Question: Help with #41 based on previous question #40 40. For a particular atom, the absorption of a photon of wavelength 4.67106m is necessary for atom
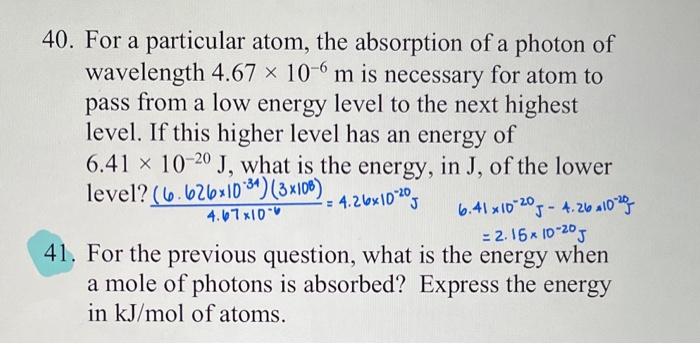
40. For a particular atom, the absorption of a photon of wavelength 4.67106m is necessary for atom to pass from a low energy level to the next highest level. If this higher level has an energy of 6.411020J, what is the energy, in J, of the lower level? 4.67106(6.6261034)(3108)=4.261020J 6.411020J4.261020J=2.151020J 41. For the previous question, what is the energy when a mole of photons is absorbed? Express the energy in kJ/mol of atoms
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


