Question: help with part b please! (a) Show that (b) 1 mole of Van der Waals gas is expanded at constant temperature from pressure and molar
help with part b please! 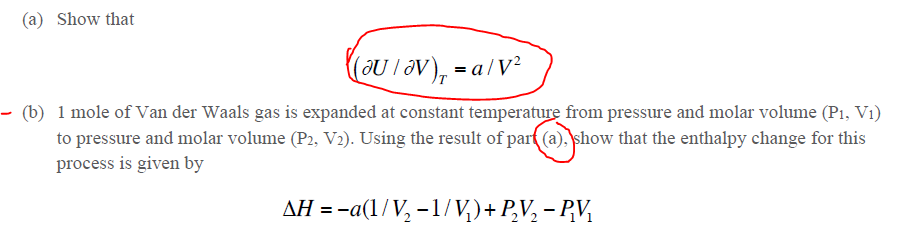
(a) Show that (b) 1 mole of Van der Waals gas is expanded at constant temperature from pressure and molar volume (P1,V1) to pressure and molar volume (P2,V2). Using the result of par(a),) show that the enthalpy change for this process is given by H=a(1/V21/V1)+P2V2P1V1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


