Question: Help with question 2! 1. Prior to lab, print Table 1 and do the following: A. Determine whether each substance is polar or nonpolar. B.
Help with question 2!
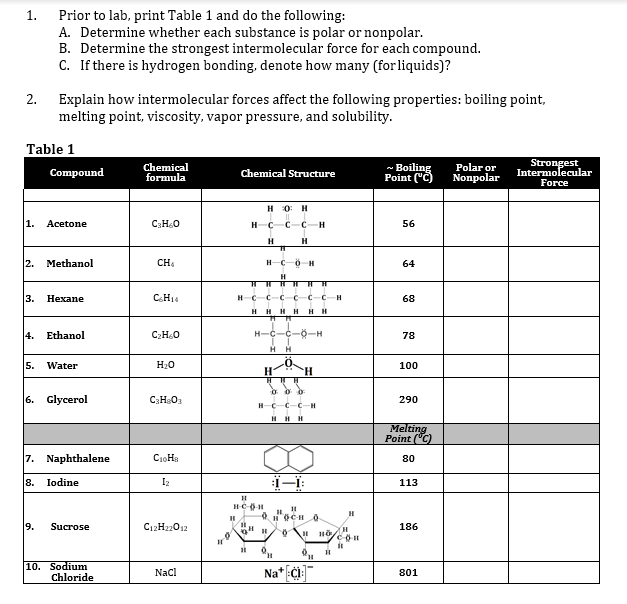
1. Prior to lab, print Table 1 and do the following: A. Determine whether each substance is polar or nonpolar. B. Determine the strongest intermolecular force for each compound. C. If there is hydrogen bonding, denote how many (forliquids)? 2. Explain how intermolecular forces affect the following properties: boiling point, melting point, viscosity, vapor pressure, and solubility
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


