Question: help with solving this please? -33 3 points If the combustion of a quantity of ethyne (CH) in an excess of oxygen produces a 1.32
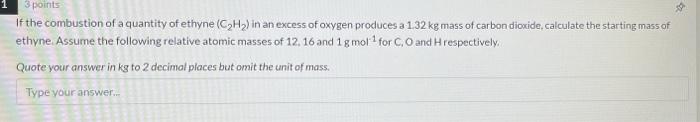
-33 3 points If the combustion of a quantity of ethyne (CH) in an excess of oxygen produces a 1.32 kg mass of carbon dioxide, calculate the starting mass of ethyne Assume the following relative atomic masses of 12, 16 and 1 g mola for CO and respectively Quote your answer in kg to 2 decimal places but omit the unit of mass. Type your
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


