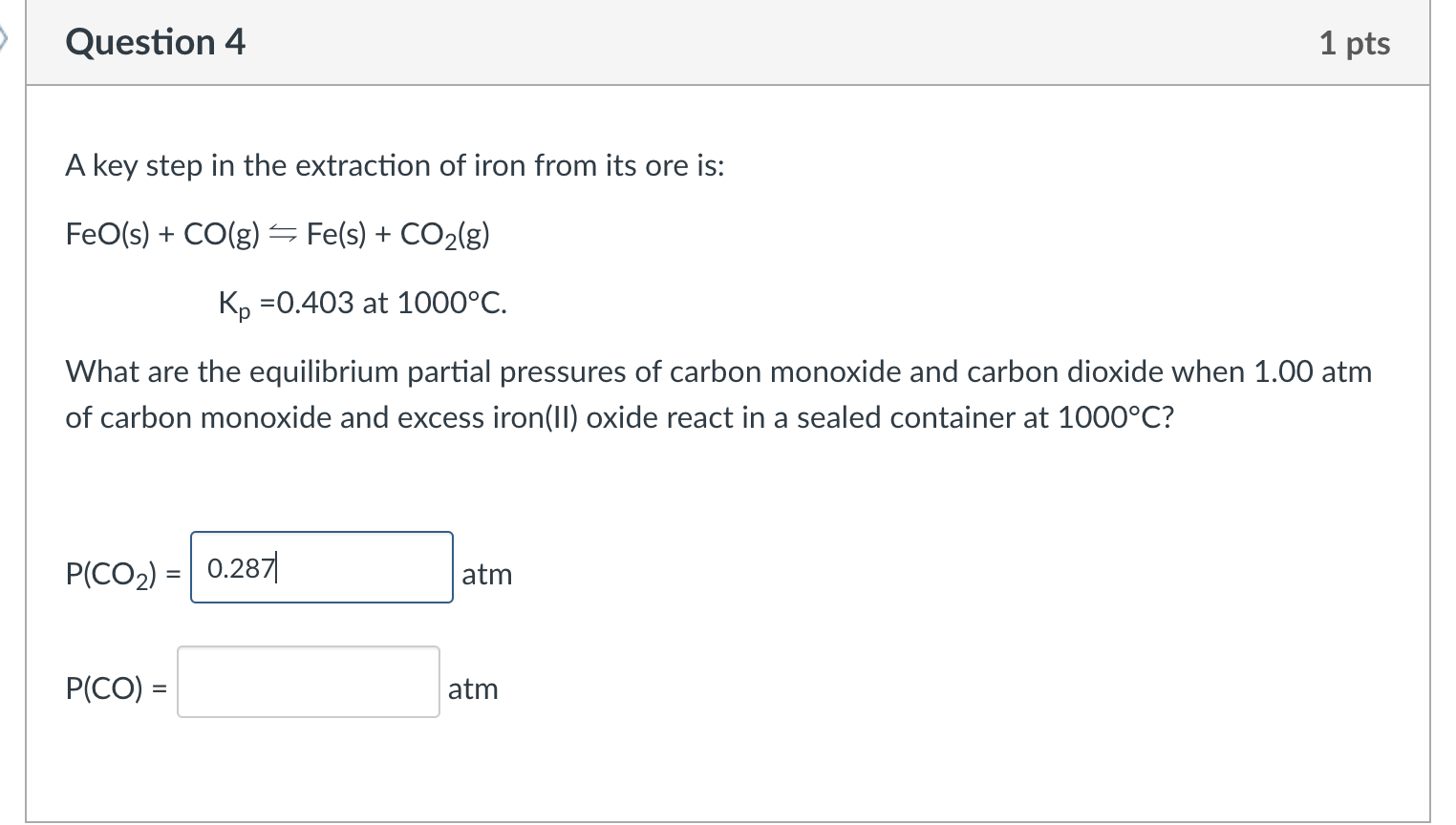
Question: Help with this question please A key step in the extraction of iron from its ore is: FeO(s)+CO(g)KpFe(s)+CO2(g)=0.403at1000C. What are the equilibrium partial pressures of
Help with this question please
A key step in the extraction of iron from its ore is: FeO(s)+CO(g)KpFe(s)+CO2(g)=0.403at1000C. What are the equilibrium partial pressures of carbon monoxide and carbon dioxide when 1.00atm of carbon monoxide and excess iron(II) oxide react in a sealed container at 1000C ? P(CO2)=atm P(CO)=atm
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


