Question: Help witn 1 & 2 pleasee 1. Complete the following table, using your actual measured amounts. Greyed-out cells do not need to be filled. x1.0612y135.169mol1mol1mol1000mol=8823102.8991mol1mol1000mol1.1=.07x=crudemRecrystalizmp:167
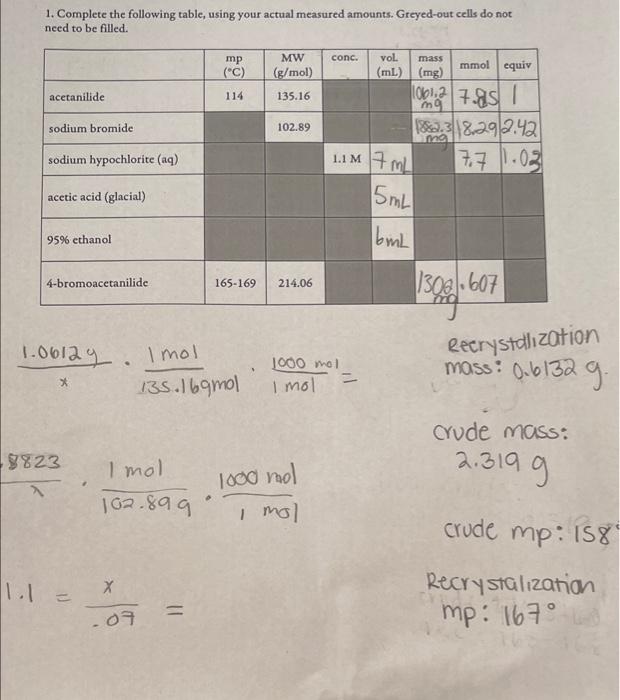

1. Complete the following table, using your actual measured amounts. Greyed-out cells do not need to be filled. x1.0612y135.169mol1mol1mol1000mol=8823102.8991mol1mol1000mol1.1=.07x=crudemRecrystalizmp:167 a) NaBr(aq)+NaClO(aq)Br2(l)+NaCl(aq)+H2O (need to balance a redox reaction!) b) 2. Based on your above answers, and your measured amounts, what was the limiting reactant for your reaction? 3. Based on your above answer, calculate: a) the theoretical yield for the reaction b) the percent yield that you obtained
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


