Question: helpp As a 1st year PhD student learning how to polymerize methyl acrylate, you try your hand at planning a polymerization experiment. You choose AIBN
helpp
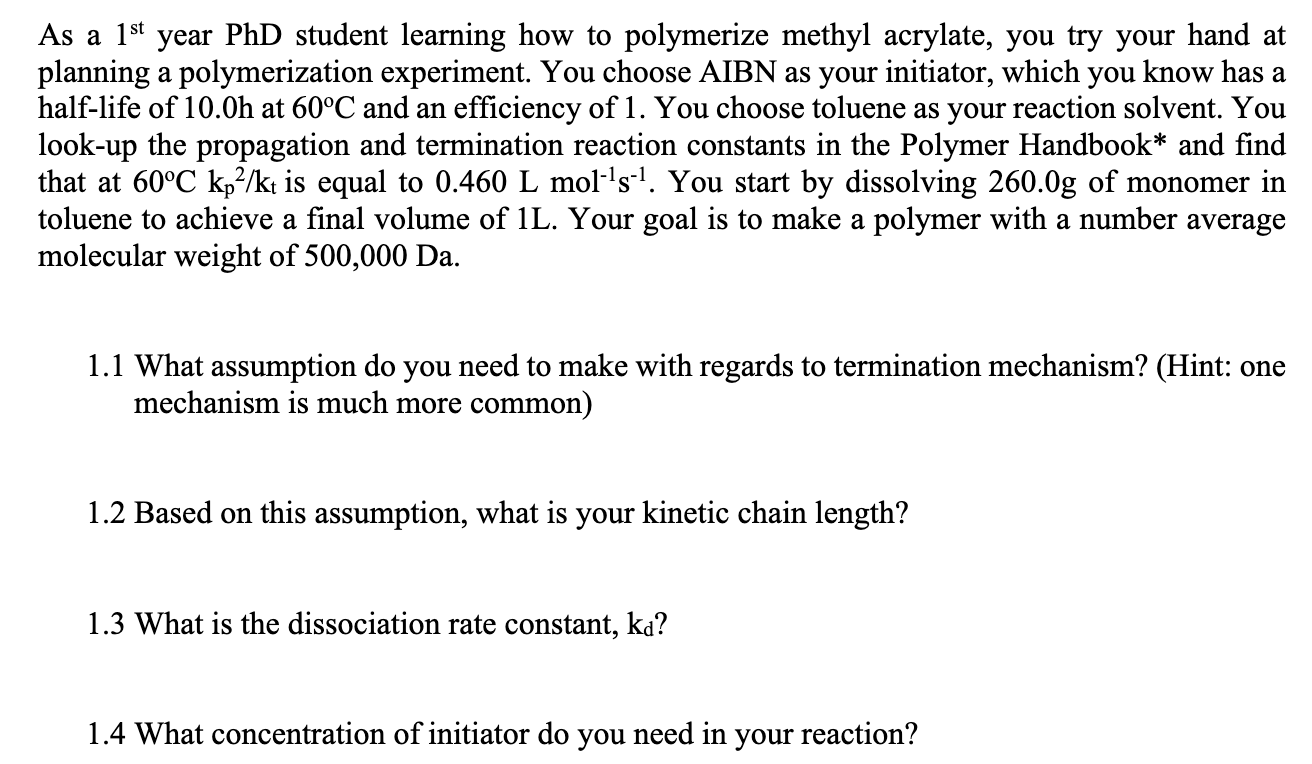
As a 1st year PhD student learning how to polymerize methyl acrylate, you try your hand at planning a polymerization experiment. You choose AIBN as your initiator, which you know has a half-life of 10.0h at 60C and an efficiency of 1. You choose toluene as your reaction solvent. You look-up the propagation and termination reaction constants in the Polymer Handbook* and find that at 60C kp-/kt is equal to 0.460 L mol-'s-1. You start by dissolving 260.0g of monomer in toluene to achieve a final volume of 1L. Your goal is to make a polymer with a number average molecular weight of 500,000 Da. 1.1 What assumption do you need to make with regards to termination mechanism? (Hint: one mechanism is much more common) 1.2 Based on this assumption, what is your kinetic chain length? 1.3 What is the dissociation rate constant, ka? 1.4 What concentration of initiator do you need in your reaction? As a 1st year PhD student learning how to polymerize methyl acrylate, you try your hand at planning a polymerization experiment. You choose AIBN as your initiator, which you know has a half-life of 10.0h at 60C and an efficiency of 1. You choose toluene as your reaction solvent. You look-up the propagation and termination reaction constants in the Polymer Handbook* and find that at 60C kp-/kt is equal to 0.460 L mol-'s-1. You start by dissolving 260.0g of monomer in toluene to achieve a final volume of 1L. Your goal is to make a polymer with a number average molecular weight of 500,000 Da. 1.1 What assumption do you need to make with regards to termination mechanism? (Hint: one mechanism is much more common) 1.2 Based on this assumption, what is your kinetic chain length? 1.3 What is the dissociation rate constant, ka? 1.4 What concentration of initiator do you need in your reaction
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


