Question: helpppp:(((( When the following equation is balanced properly under basic conditions, what are the coefficients of the species shown? Ag+CrO42Ag2O+Cr(OH)3 Water appears in the balanced
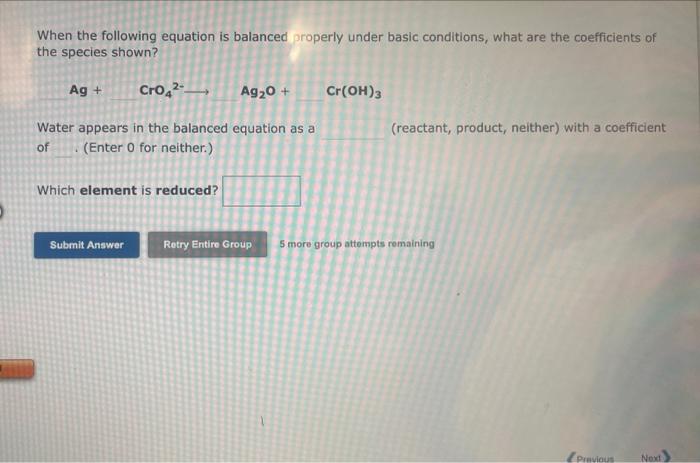
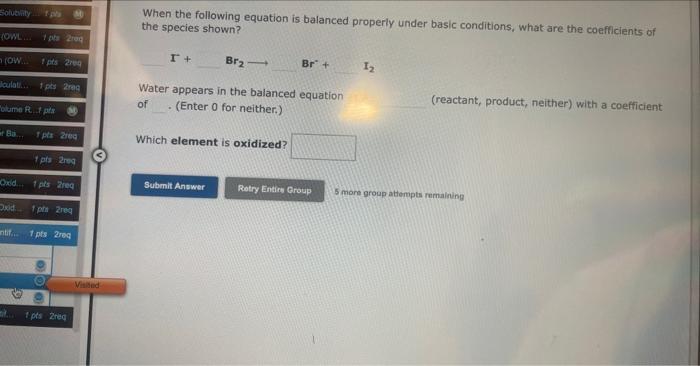
When the following equation is balanced properly under basic conditions, what are the coefficients of the species shown? Ag+CrO42Ag2O+Cr(OH)3 Water appears in the balanced equation as a (reactant, product, neither) with a coefficient (Enter 0 for neither.) Which element is reduced? 5 more group attempts remaining When the following equation is balanced properly under basic conditions, what are the coefficients of the species shown? Water appears in the balanced equation of (Enter 0 for neither.) (reactant, product, neither) with a coefficient Which element is oxidized? 5 mome group attempts renalining
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


