Question: Balancing Oxidation-Reduction Reactions Use the References to access important values if needed for this question. When the following equation is balanced properly under basic conditions,
Balancing Oxidation-Reduction Reactions 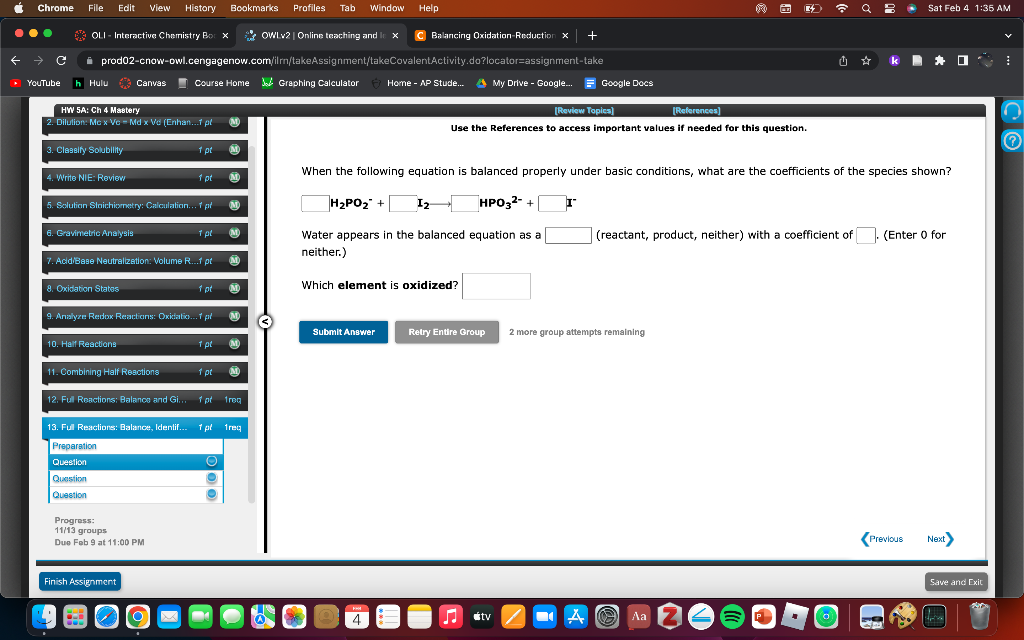
Use the References to access important values if needed for this question. When the following equation is balanced properly under basic conditions, what are the coefficients of the species shown? H2PO2+I2HPO32+[ Water appears in the balanced equation as a (reactant, product, neither) with a coefficient of (Enter 0 for neither.) Which element is oxidized? 2 more group attempte remaining
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


