Question: here I have the solution to the problem which is correct. My question is Where does the 1.4 comes from ? (gamma=1.4) where do I

here I have the solution to the problem which is correct. My question is Where does the 1.4 comes from ? (gamma=1.4) where do I get that value ???? plz help explaining in detail.
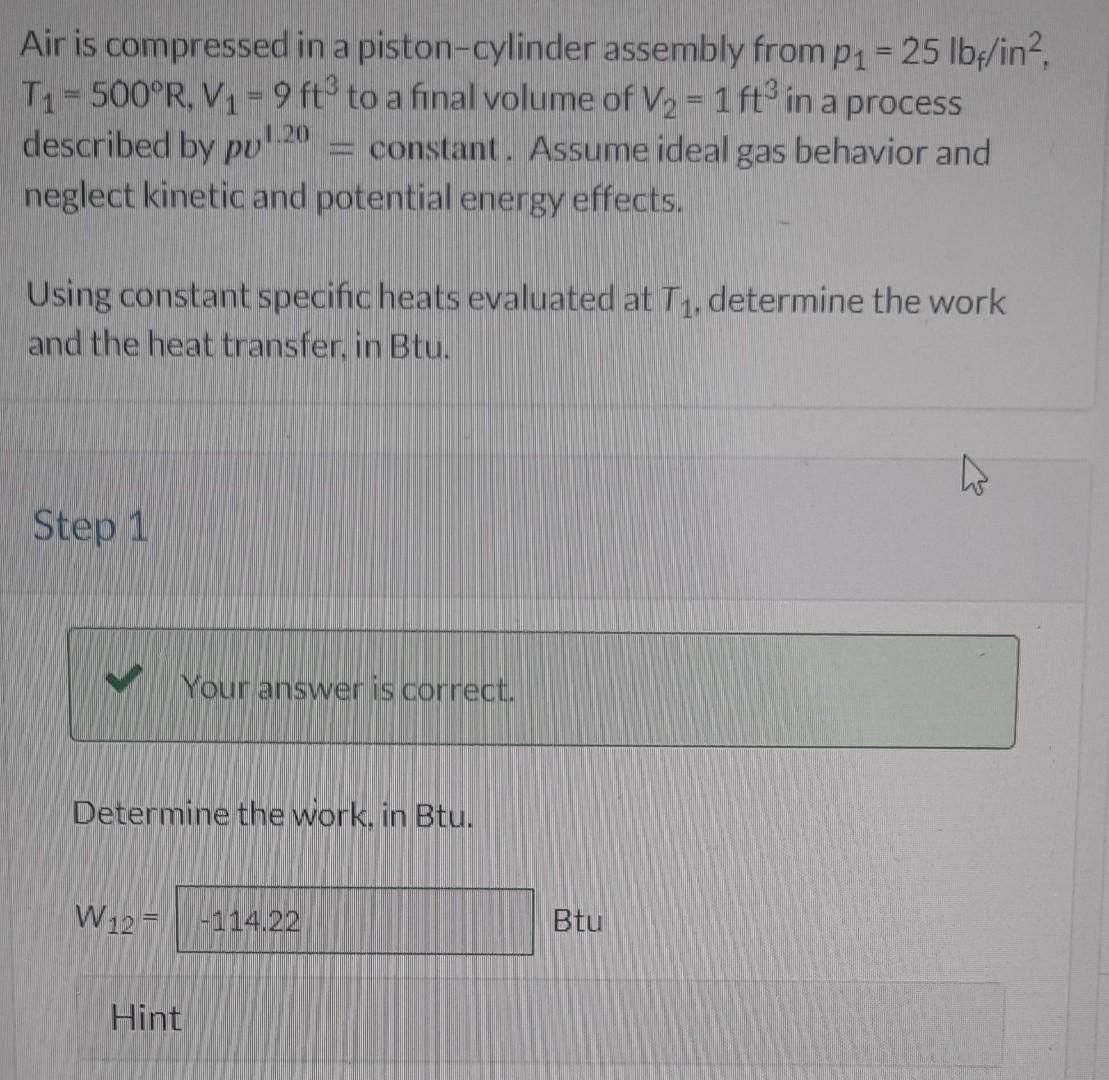
Air is compressed in a piston-cylinder assembly from p1=25lbf/in2, T1=500R,V1=9ft3 to a final volume of V2=1ft3 in a process described by pv1.20= constant. Assume ideal gas behavior and neglect kinetic and potential energy effects. Using constant specific heats evaluated at T1, determine the work and the heat transfer, in Btu. Step 1 Determine the work, in Btu. W12= Hint Qe Given:- P1V1T1=25(bf/in2;P2=?=9ft3=15552in3;V2=1ft3=1728in3=500RP1V11.2=P2V21.2P2=P1[V2V1]1.2=25[19]P2=349.165lbflin2 i) Work Transfer:- W=n11[P1V1P2V2]W=1.211[2515552349.1651728]W=1072785.6lbfin W=114.88B+4 ii) Heat Transfer:- Q=1nn1P1V1P2V2Q=1nW=1.411.41.2114.88Q=57.44B+4Ans
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


