Question: Here is an example to do the self test in the second photo at th end Book is atkins edition 11 Physical chemistry answer is
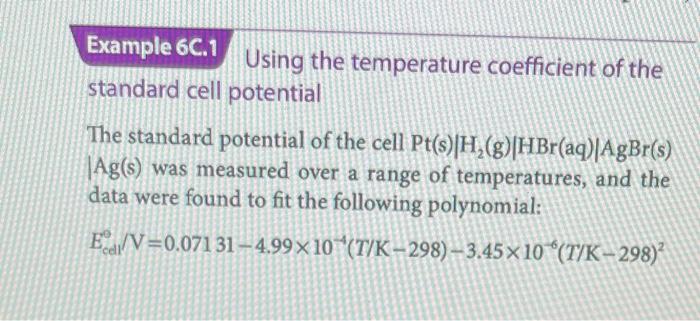

Using the temperature coefficient of the standard cell potential The standard potential of the cell Pt(s)H2(g)HBr(aq)AgBr(s) Ag(s) was measured over a range of temperatures, and the data were found to fit the following polynomial: Eccl0/V=0.071314.99104(T/K298)3.45106(T/K298)2 The cell reaction is AgBr(s)+21H2(g)Ag(s)+HBr(ag). and has v=1. Evaluate the standard reaction Gibbs energy. enthalpy, and entropy at 298K. Collect your thoughts The standard Gibbs energy of reaction is obtained by using eqn 6 C.3 after evaluating Ea at 298K and by using 1VC=1J. The standard reaction entropy is obtained by using eqn 6C.6, which involves differentiating the polynomial with respect to T and then setting T=298K. The standard reaction enthalpy is obtained by combining the values of the standard Gibbs energy and entropy. The solution At T=298K,Ecal=0.07131V, so G=vFEeih=(1)(9.6485104Cmol1)(0.07131V)=6.880103CVmol1=6.880kJmol1 The temperature coefficient of the standard cell potential is dTdEade=4.99104VK12(3.45106)(T/K298)VK1 At T=298K this expression evaluates to dTdEe0=4.99104VK1 So, from eqn 6 C. 6 the standard reaction entropy is S=vFdTdEct0=(1)(9.6485104Cmol1)(4.99104VK1)=48.1JK1mol1 The negative value stems in part from the elimination of gas in the cell reaction. It then follows that TH=tG6+T,S6=6.880kJmol1+(298K)(0.0481kJK1mol1)=21.2kJmol1 Comment. One difficulty with this procedure lies in the accurate measurement of small temperature coefficients of cell potential. Nevertheless, it is another example of the striking ability of thermodynamics to relate the apparently unrelated, in this case to relate electrical measurements to thermal properties. Self-test 6C.1 Predict the standard potential of the Harned cell, Pt(s)H2(g)HCl(aq)AgClg(s)Ag(s), at 303K from tables of thermodynamic data. Actecotineruy Using the temperature coefficient of the standard cell potential The standard potential of the cell Pt(s)H2(g)HBr(aq)AgBr(s) Ag(s) was measured over a range of temperatures, and the data were found to fit the following polynomial: Eccl0/V=0.071314.99104(T/K298)3.45106(T/K298)2 The cell reaction is AgBr(s)+21H2(g)Ag(s)+HBr(ag). and has v=1. Evaluate the standard reaction Gibbs energy. enthalpy, and entropy at 298K. Collect your thoughts The standard Gibbs energy of reaction is obtained by using eqn 6 C.3 after evaluating Ea at 298K and by using 1VC=1J. The standard reaction entropy is obtained by using eqn 6C.6, which involves differentiating the polynomial with respect to T and then setting T=298K. The standard reaction enthalpy is obtained by combining the values of the standard Gibbs energy and entropy. The solution At T=298K,Ecal=0.07131V, so G=vFEeih=(1)(9.6485104Cmol1)(0.07131V)=6.880103CVmol1=6.880kJmol1 The temperature coefficient of the standard cell potential is dTdEade=4.99104VK12(3.45106)(T/K298)VK1 At T=298K this expression evaluates to dTdEe0=4.99104VK1 So, from eqn 6 C. 6 the standard reaction entropy is S=vFdTdEct0=(1)(9.6485104Cmol1)(4.99104VK1)=48.1JK1mol1 The negative value stems in part from the elimination of gas in the cell reaction. It then follows that TH=tG6+T,S6=6.880kJmol1+(298K)(0.0481kJK1mol1)=21.2kJmol1 Comment. One difficulty with this procedure lies in the accurate measurement of small temperature coefficients of cell potential. Nevertheless, it is another example of the striking ability of thermodynamics to relate the apparently unrelated, in this case to relate electrical measurements to thermal properties. Self-test 6C.1 Predict the standard potential of the Harned cell, Pt(s)H2(g)HCl(aq)AgClg(s)Ag(s), at 303K from tables of thermodynamic data. Actecotineruy
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


