Question: the example to do the self test who is located at the end Book is atkins edition 11 Physical chemistry answer is +0.071 V The
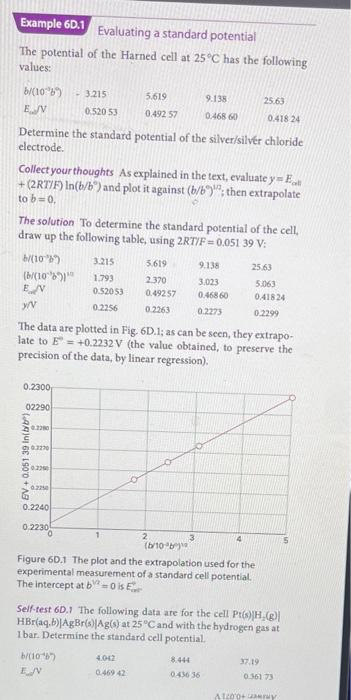
The potential of the Harned cell at 25C has the following values: Determine the standard potential of the silver/silver chloride electrode. Collect your thoughts As explained in the text, evaluate y=Eoal. +(2RT/F)ln(b/b) and plot it against (b/b/1; then extrapolate to b=0. The solution To determine the standard potential of the cell, draw up the following table, using 2RT/F=0.05139V : The data are plotted in Fig. 6D.1; as can be seen, they extrapolate to E=+0.2232V (the value obtained, to preserve the precision of the data, by linear regression). Figure 60.1 The plot and the extrapolation used for the experimental measurement of a standard cell potential. The intercept a1b2=0 is Ei Self-test 6D.1 The following data are for the cell Pt(s)H2(g) HBr(aq,b)AgBr(B)Ag(s) at 25C and with the hydrogen gas at I bar. Determine the standard cell potential. b/(101b7E.jV4.0620.469428.4440.4363637.190.36173
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


