Question: How do I do Part B and Part C? Submit Previous Answers Learning Goal: To learn how graphs can be used to answer kinetics questions.
How do I do Part B and Part C?
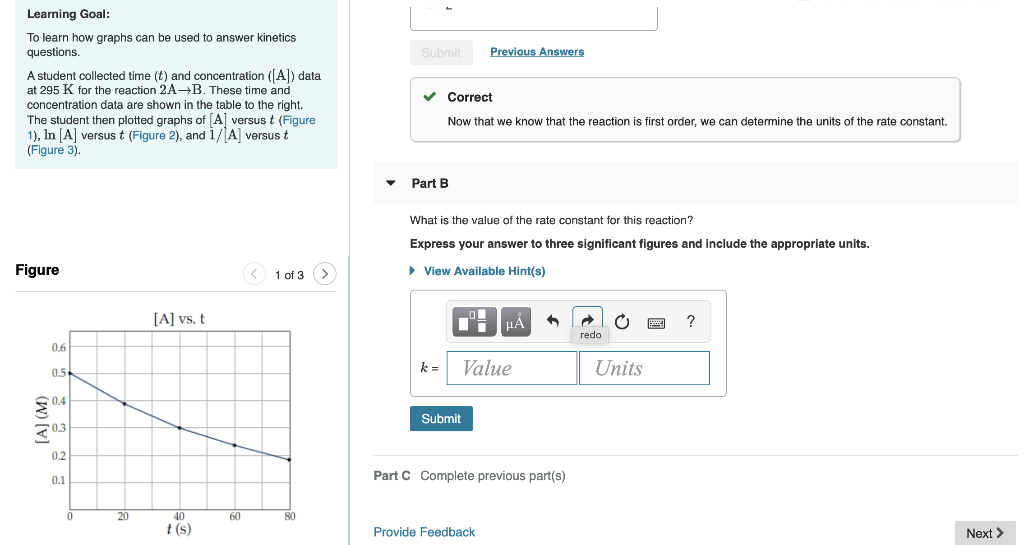
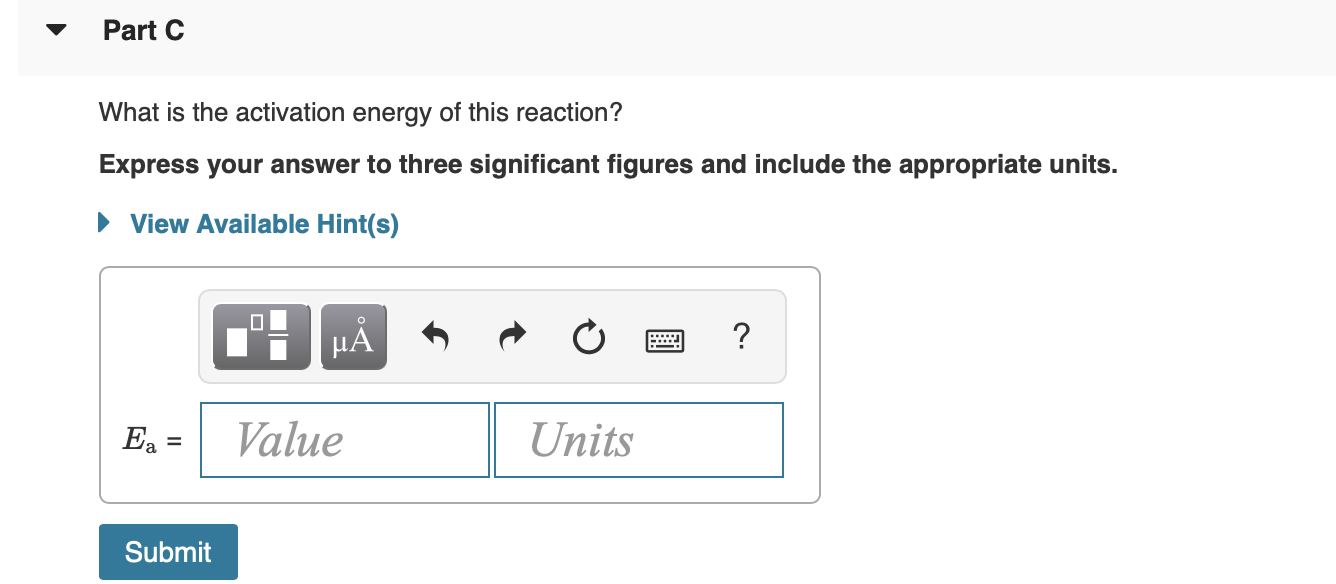
Submit Previous Answers Learning Goal: To learn how graphs can be used to answer kinetics questions. A student collected time (t) and concentration ([A]) data at 295 K for the reaction 2A-B. These time and concentration data are shown in the table to the right. The student then plotted graphs of Al versus t (Figure 1), In [A] versus t (Figure 2), and i/[A] versust (Figure 3). Correct Now that we know that the reaction is first order, we can determine the units of the rate constant. Part B What is the value of the rate constant for this reaction? Express your answer to three significant figures and include the appropriate units. Figure 1 of 3 > View Available Hint(s) [A] vs. t ? redo 0.6 Value 0.5 Units 0.4 [A] (M) Submit 0.3 0.2 0.1 Part C Complete previous part(s) 0 20 60 80 t(s) Provide Feedback Next > Part C What is the activation energy of this reaction? Express your answer to three significant figures and include the appropriate units. View Available Hint(s) ? Ea = Value Units Submit
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


