Question: hey can you help me Question 9 (1.5 points) Nitrosyl chloride gas decomposes according to the chemical equation below. A RICE table is prepared in
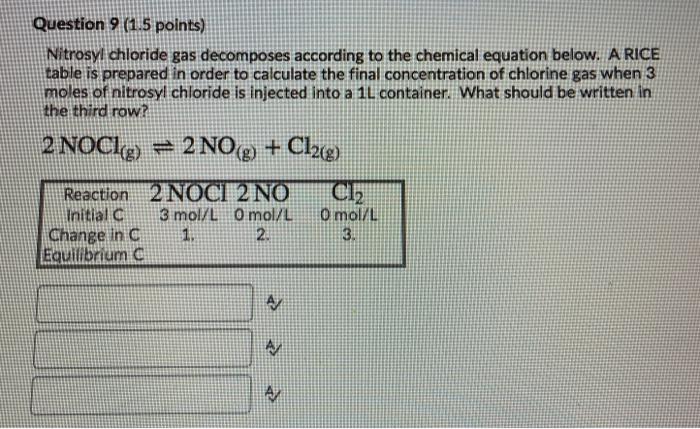
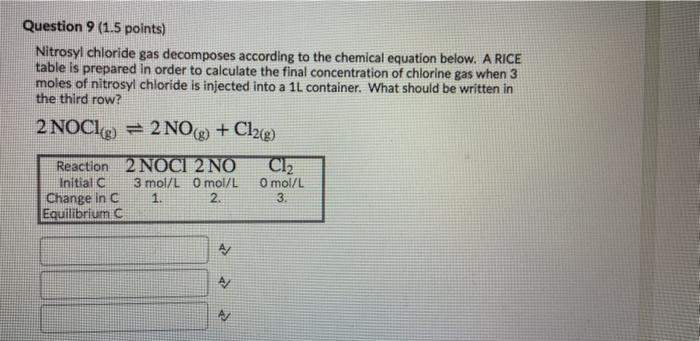
Question 9 (1.5 points) Nitrosyl chloride gas decomposes according to the chemical equation below. A RICE table is prepared in order to calculate the final concentration of chlorine gas when 3 moles of nitrosyl chloride is injected into a 1L container. What should be written in the third row? 2 NOC1g) = 2 NO + Cl2(g) Cl2 Reaction 2 NOCI 2 NO Initiala 3 mol/L O mol/L Change in C 1. 2. Equilibrium C Omol/L 3. A AM Question 9 (1.5 points) Nitrosyl chloride gas decomposes according to the chemical equation below. A RICE table is prepared in order to calculate the final concentration of chlorine gas when 3 moles of nitrosyl chloride is injected into a 1L container. What should be written in the third row? 2 NOCL9 = 2 NO(g) + Cl2(g) Reaction 2 NOCI 2 NO Cl2 Initial 3 mol/L O mol/L O mol/L Change in C 1. 2. 3. Equilibrium A A/
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


