Question: Hey, I'm super lost with this question, I've never come across this problem before and I don't ever recall doing this in class. Any help
Hey, I'm super lost with this question, I've never come across this problem before and I don't ever recall doing this in class.
Any help with this question would be appreciated!
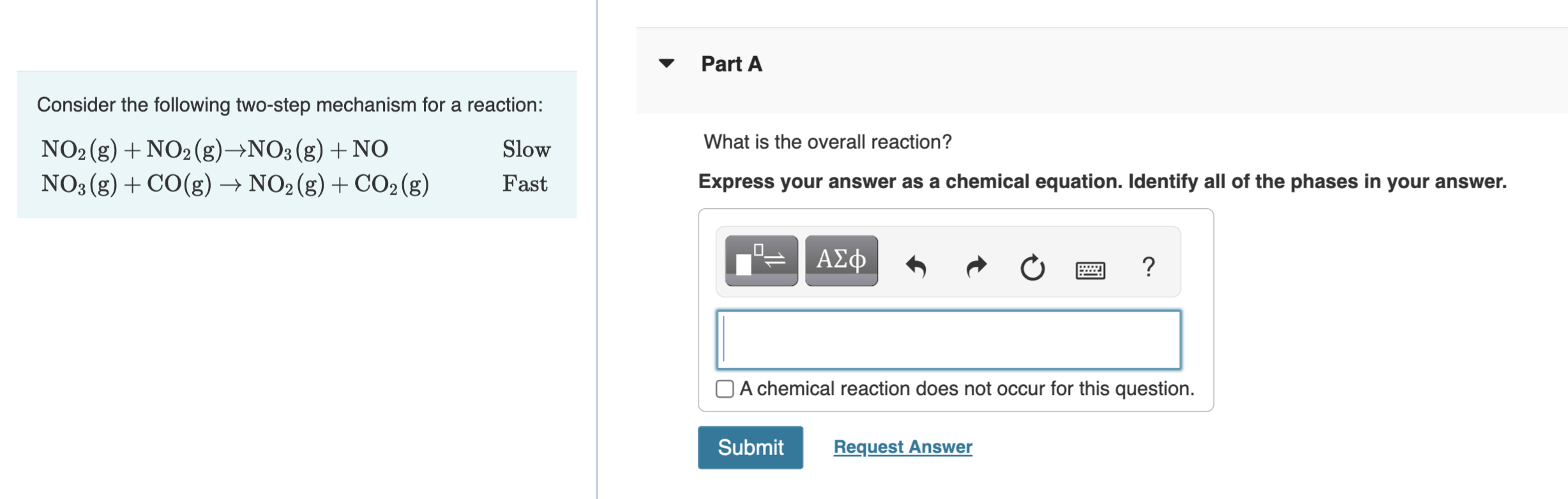
Consider the following two-step mechanism for a reaction: NO2(g)+NO2(g)NO3(g)+NONO3(g)+CO(g)NO2(g)+CO2(g)SlowFast What is the overall reaction? Express your answer as a chemical equation. Identify all of the phases in your
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


