Question: Determine AH in kJ for the following reaction using Hess' Law You MUST use Hess' Law to receive any credit for this problem. 3C
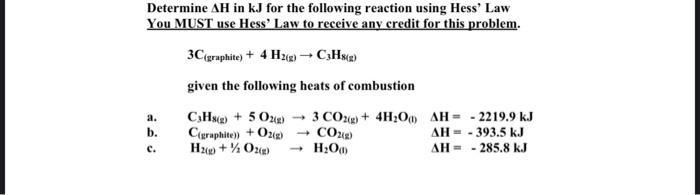
Determine AH in kJ for the following reaction using Hess' Law You MUST use Hess' Law to receive any credit for this problem. 3C (graphite) + 4H2(g) C3H8(2) given the following heats of combustion C3Hs()+ 5 O2(g) 3 CO2(g) + 4HO) C(graphite)) + O2(g) CO2(g) H(g) + O2(g) HO) - a. b. C. 2219.9 kJ AH = AH = -393.5 kJ AH = 285.8 kJ
Step by Step Solution
3.48 Rating (158 Votes )
There are 3 Steps involved in it
To determine the enthalpy change H for the target reaction using Hesss Law we must manipulate the gi... View full answer

Get step-by-step solutions from verified subject matter experts


