Question: Hi can someone pleaseeeee help me Answer this. Please try and answer it on paper, thank you. 2:22 AM Tue Dec 3 16% c30_m8_106_assignment 2
Hi can someone pleaseeeee help me Answer this. Please try and answer it on paper, thank you.
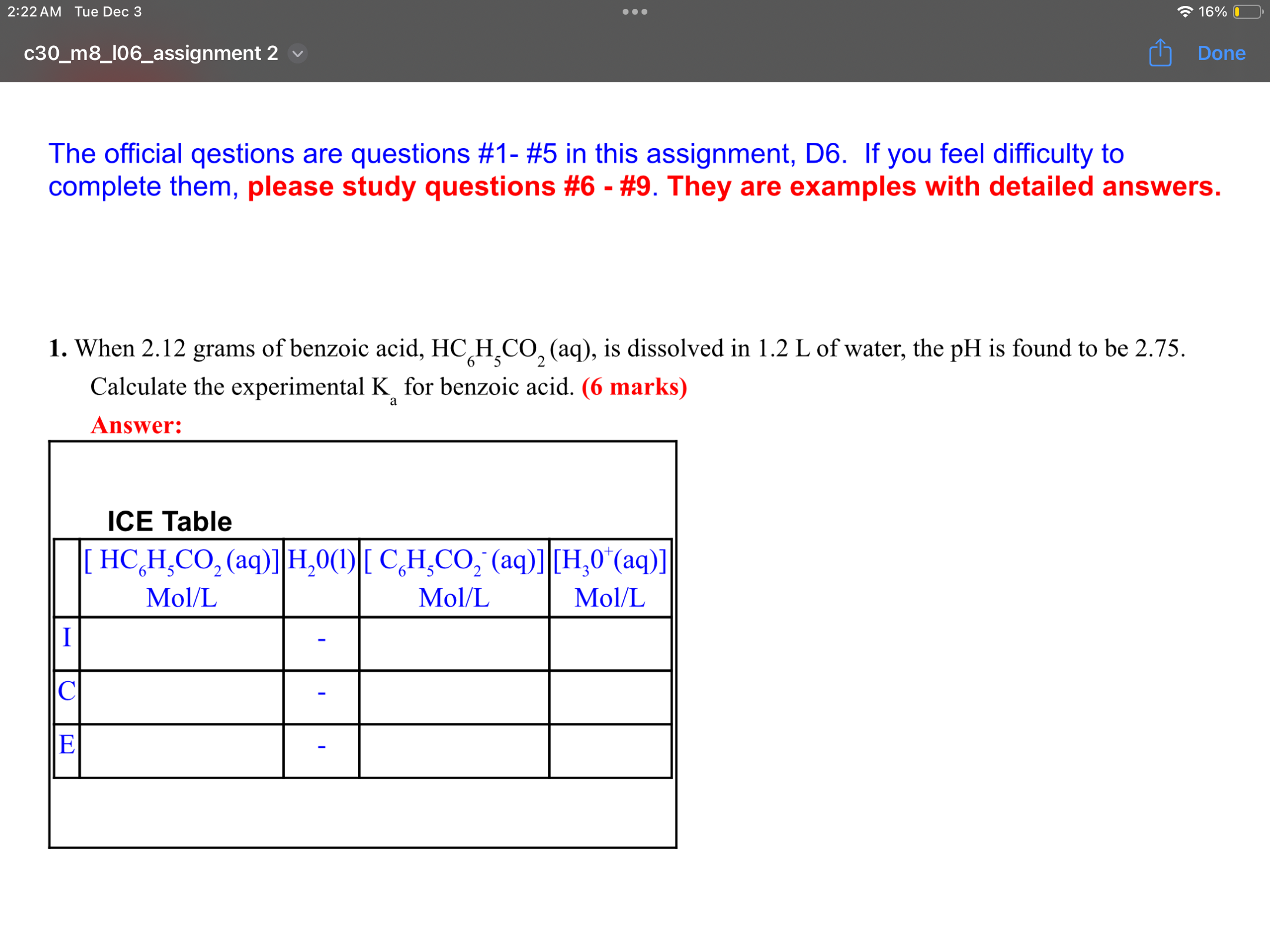
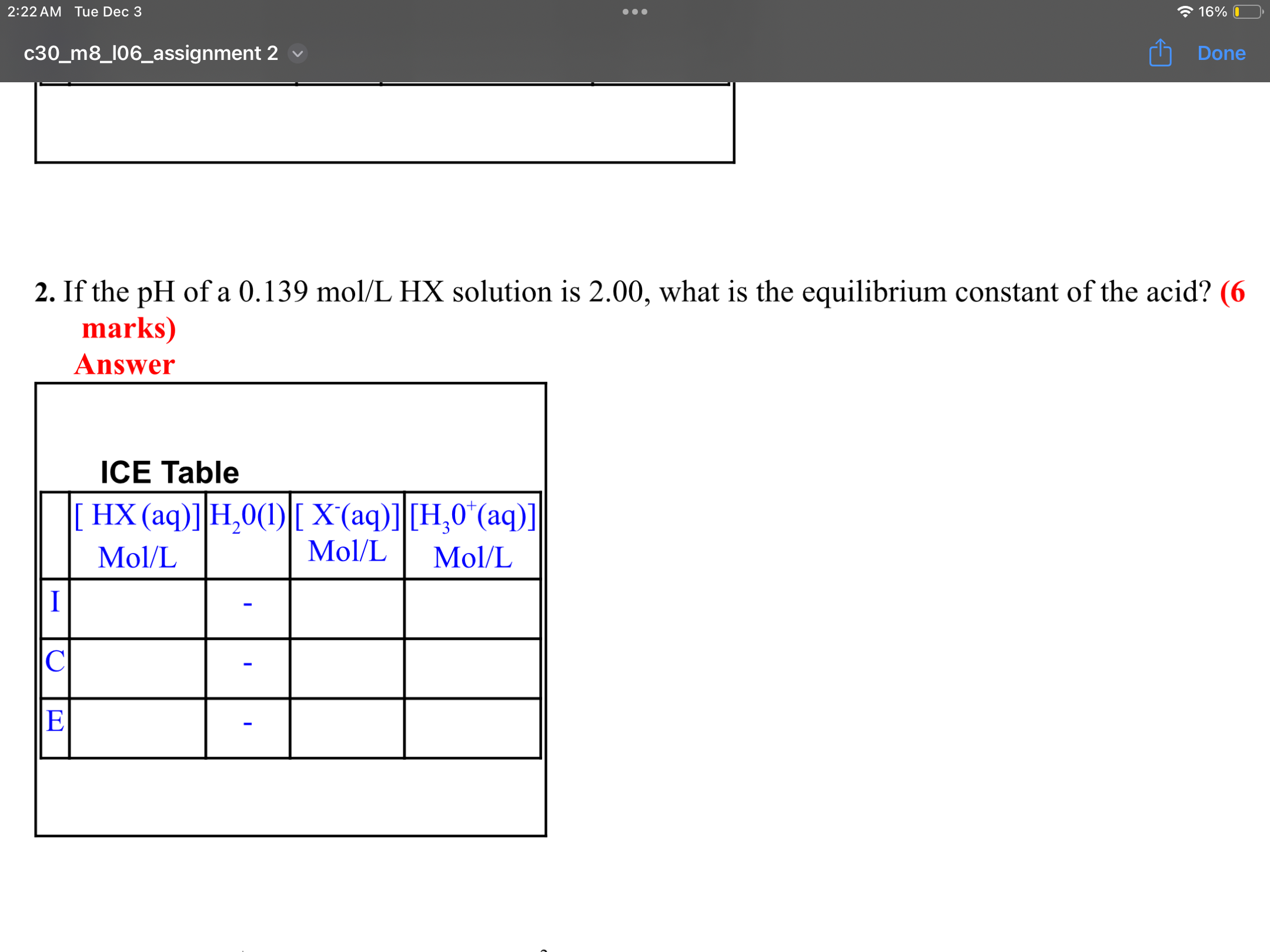
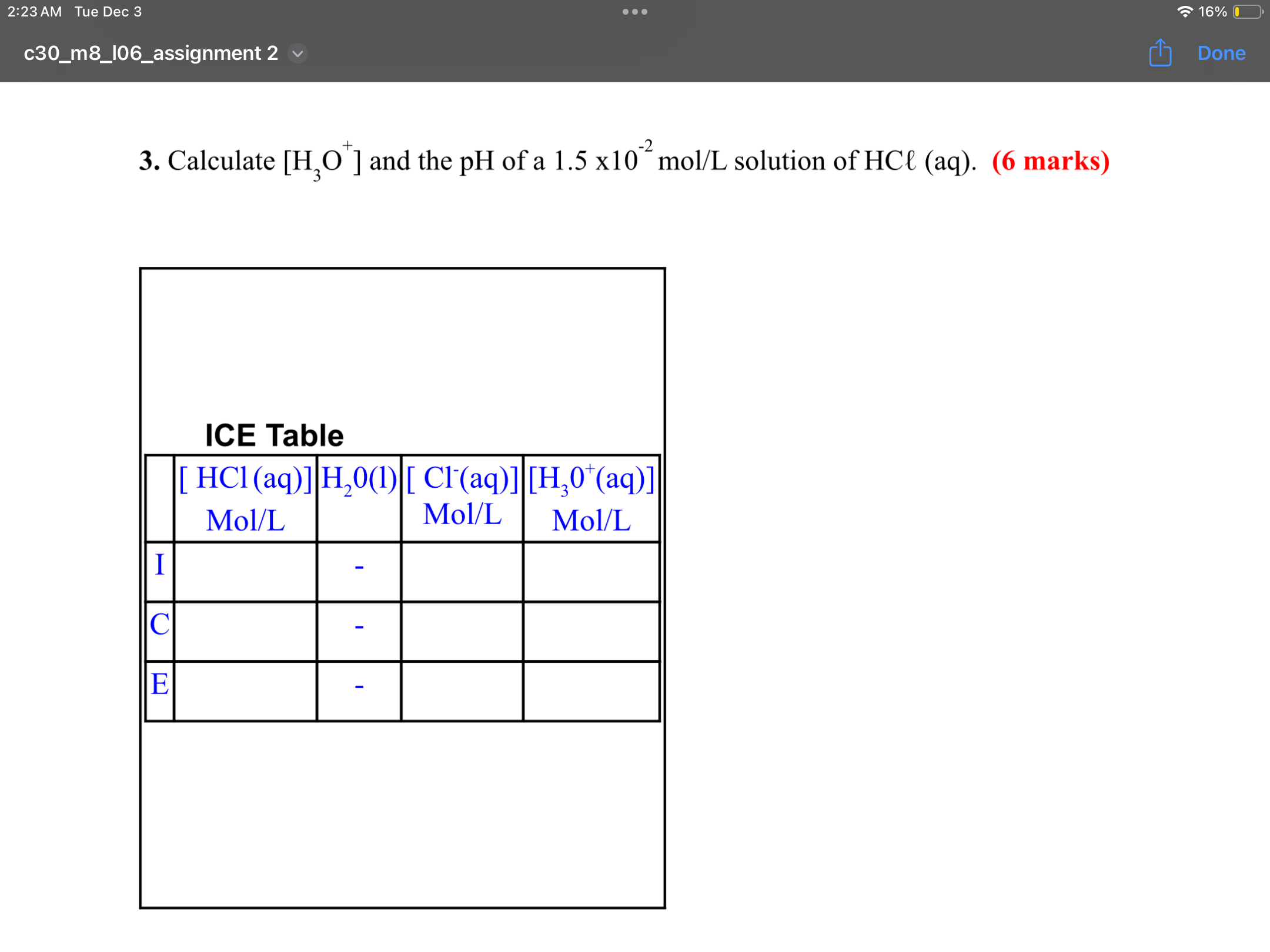
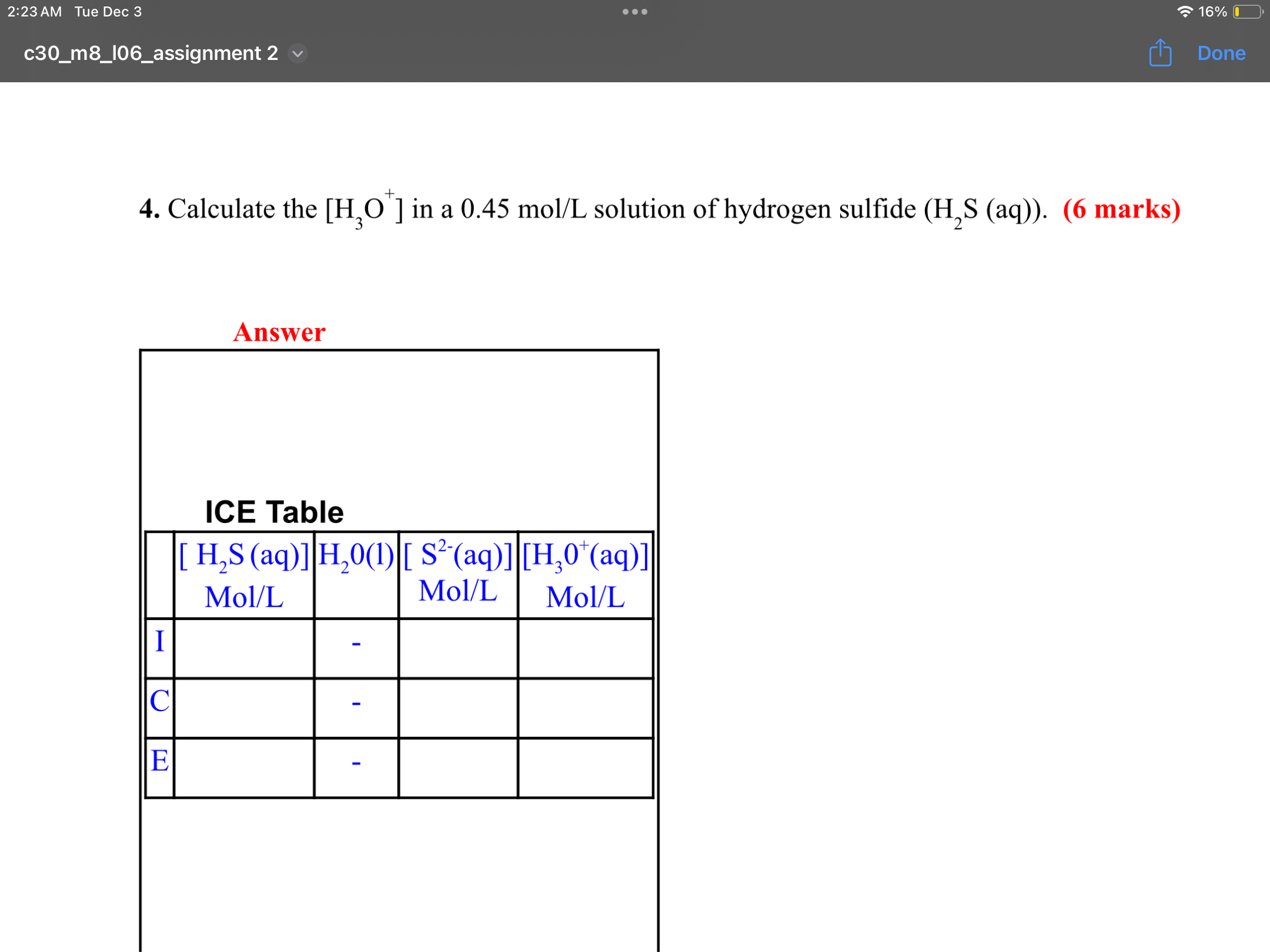
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


