Question: hi, can you help me with this question? step-by-step solution is appreciated. thank you 3. a) The vapor pressure of each component in a mixture
hi, can you help me with this question? step-by-step solution is appreciated. thank you 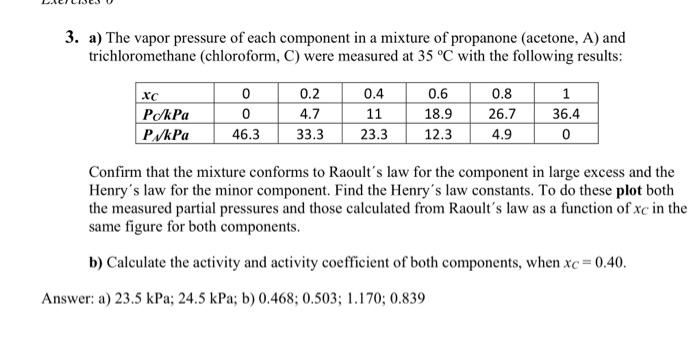

3. a) The vapor pressure of each component in a mixture of propanone (acetone, A) and trichloromethane (chloroform, C) were measured at 35C with the following results: Confirm that the mixture conforms to Raoult's law for the component in large excess and the Henry's law for the minor component. Find the Henry's law constants. To do these plot both the measured partial pressures and those calculated from Raoult's law as a function of xC in the same figure for both components. b) Calculate the activity and activity coefficient of both components, when xC=0.40. a) 23.5kPa;24.5kPa; b) 0.468;0.503;1.170;0.839
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


