Question: Please I really need help with the total vapor question. Thank you! 3. A solution contains 22.4 g glucose (C6H120.) dissolved in 0.500 L of
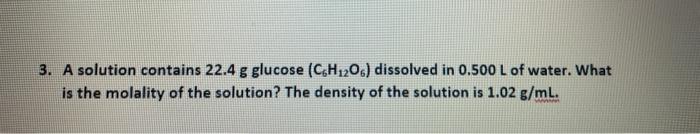

3. A solution contains 22.4 g glucose (C6H120.) dissolved in 0.500 L of water. What is the molality of the solution? The density of the solution is 1.02 g/mL. 2. A solution is a 2:1 mixture of two volatile components A and B. Pure B has a vapor pressure double of the vapor pressure of pure A. The total vapor pressure of the solution equals to
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


