Question: Hi, I have a problem in mass balance that involves optimization using excel . It is attached in this post. The process depicted above is
Hi,
I have a problem in mass balance that involves optimization using excel. It is attached in this post.
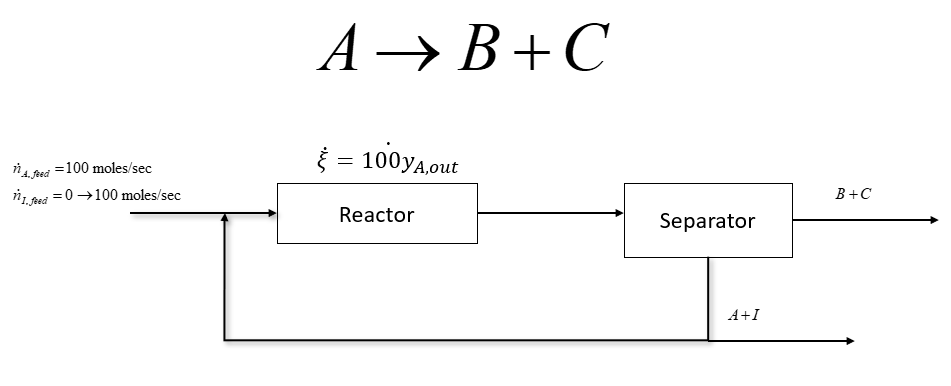
The process depicted above is used to react Component A into Components B and C. In the process the feed stream which can contain some inert component, I, is mixed with a recycle stream before entering the reactor. In the reactor, the reaction extent is given in the process diagram which is a function of the outlet mole fraction of component A. After the reactor, a separator is used to separate components B and C from unreacted A and I. Some of A and I are recycled back to the beginning of the process and the rest is purged.
The cost of component A is $0.25/mole. There is no cost for the inert compound. The value of component B is $1.00/mole and that of component C is $0.80/mole. There is a cost of recycling the unreacted A and I, which is $0.20/mole of A and I combined. Vary the amount of inert in the feed stream and show how much A and I should be recycled to maximize the profit of the plant.
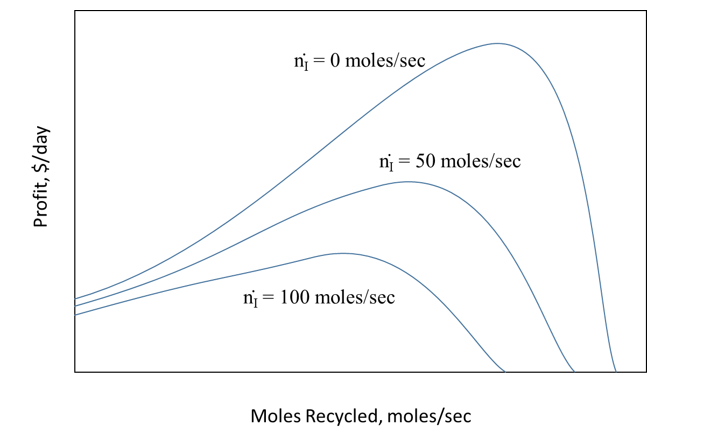
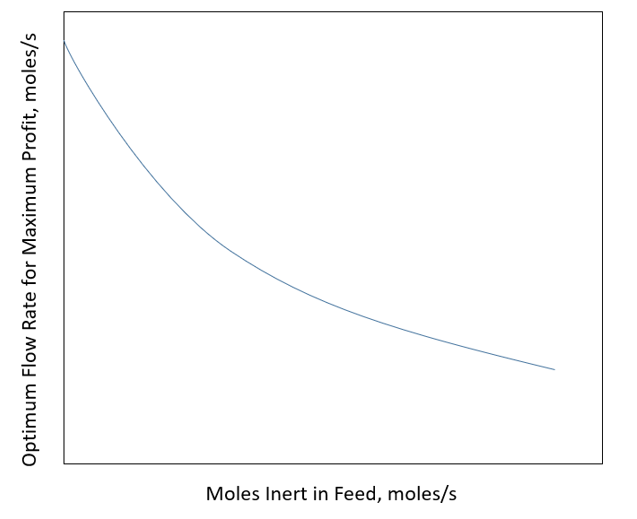
Please can anyone help? Thank you in advance!
A B+C = 100y4,out = 4. 1. Sed =100 moles/sec 71. feed = 0 +100 moles/sec B+C Reactor Separator 4+1 ni = 0 moles/sec ni = 50 moles/sec Profit, $/day ni = 100 moles/sec Moles Recycled, moles/sec Optimum Flow Rate for Maximum Profit, moles/s Moles Inert in Feed, moles/s A B+C = 100y4,out = 4. 1. Sed =100 moles/sec 71. feed = 0 +100 moles/sec B+C Reactor Separator 4+1 ni = 0 moles/sec ni = 50 moles/sec Profit, $/day ni = 100 moles/sec Moles Recycled, moles/sec Optimum Flow Rate for Maximum Profit, moles/s Moles Inert in Feed, moles/s
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


