Question: Hi, I have a question regarding reaction engineering problem set. I believe we are supposed to use Excel to calculate however I am having trouble
Hi, I have a question regarding reaction engineering problem set. I believe we are supposed to use Excel to calculate however I am having trouble starting the question and getting the answer. The question is attached as picture below.
The correct answer is:
12-5:
(a) 3920s; cA = 9.4 mol/L; cB = 3.75 mol/L; cC = 12.9 mol/L; cD = 1.25 mol/L
(b) fB = 0.604; cA = 7.63 mol/L; cB = 1.98 mol/L; cC = 14.7 mol/L; cD = 3.02 mol/L
Here is the question:
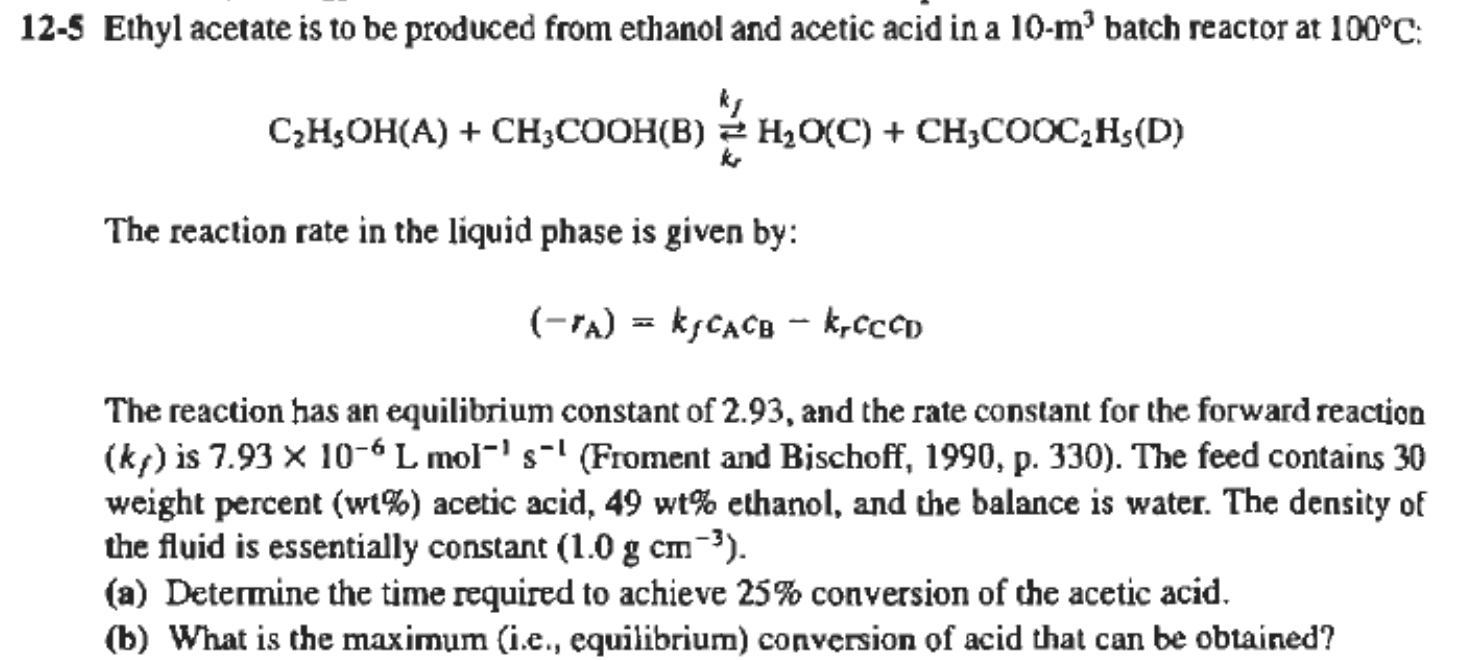
12-5 Ethyl acetate is to be produced from ethanol and acetic acid in a 10-m' batch reactor at 100C: ky C2H5OH(A) + CH3COOH(B) H2O(C) + CH3COOC2H5(D) AB2 ) The reaction rate in the liquid phase is given by: (-ra) = kjCACB - k-CCCD) The reaction has an equilibrium constant of 2.93, and the rate constant for the forward reaction (ks) is 7.93 x 10-6L mol-'s-' (Froment and Bischoff, 1990, p. 330). The feed contains 30 weight percent (wt%) acetic acid, 49 wt% ethanol, and the balance is water. The density of the fluid is essentially constant (1.0 g cm-?). (a) Determine the time required to achieve 25% conversion of the acetic acid, (b) What is the maximum (i.e., equilibrium) conversion of acid that can be obtained
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


