Question: Hi, I need help with below question A container tank initially contains oxygen gas at 600 kPa and 350 K, with a volume of 0.8
Hi, I need help with below question
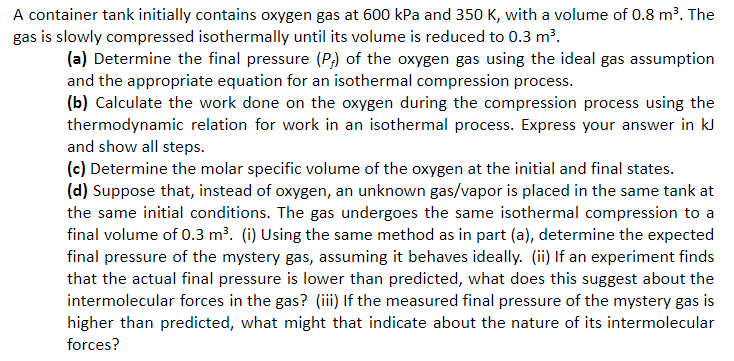 A container tank initially contains oxygen gas at 600 kPa and 350 K, with a volume of 0.8 m?. The gas is slowly compressed isothermally until its volume is reduced to 0.3 m?. (a) Determine the final pressure (P,) of the oxygen gas using the ideal gas assumption and the appropriate equation for an isothermal compression process. (b) Calculate the work done on the oxygen during the compression process using the thermodynamic relation for work in an isothermal process. Express your answer in kl] and show all steps. (c) Determine the molar specific volume of the oxygen at the initial and final states. (d) Suppose that, instead of oxygen, an unknown gas/vapor is placed in the same tank at the same initial conditions. The gas undergoes the same isothermal compression to a final volume of 0.3 m3. (i) Using the same method as in part (a), determine the expected final pressure of the mystery gas, assuming it behaves ideally. (ii) lf an experiment finds that the actual final pressure is lower than predicted, what does this suggest about the intermolecular forces in the gas? (ili) If the measured final pressure of the mystery gas Is higher than predicted, what might that indicate about the nature of its intermolecular forces
A container tank initially contains oxygen gas at 600 kPa and 350 K, with a volume of 0.8 m?. The gas is slowly compressed isothermally until its volume is reduced to 0.3 m?. (a) Determine the final pressure (P,) of the oxygen gas using the ideal gas assumption and the appropriate equation for an isothermal compression process. (b) Calculate the work done on the oxygen during the compression process using the thermodynamic relation for work in an isothermal process. Express your answer in kl] and show all steps. (c) Determine the molar specific volume of the oxygen at the initial and final states. (d) Suppose that, instead of oxygen, an unknown gas/vapor is placed in the same tank at the same initial conditions. The gas undergoes the same isothermal compression to a final volume of 0.3 m3. (i) Using the same method as in part (a), determine the expected final pressure of the mystery gas, assuming it behaves ideally. (ii) lf an experiment finds that the actual final pressure is lower than predicted, what does this suggest about the intermolecular forces in the gas? (ili) If the measured final pressure of the mystery gas Is higher than predicted, what might that indicate about the nature of its intermolecular forcesStep by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


