Question: Hi! I need help with part d please A 100. mL solution of silver chromate, Ag2CrO4 is prepared in pure water at 25C. Some of
Hi! I need help with part d please
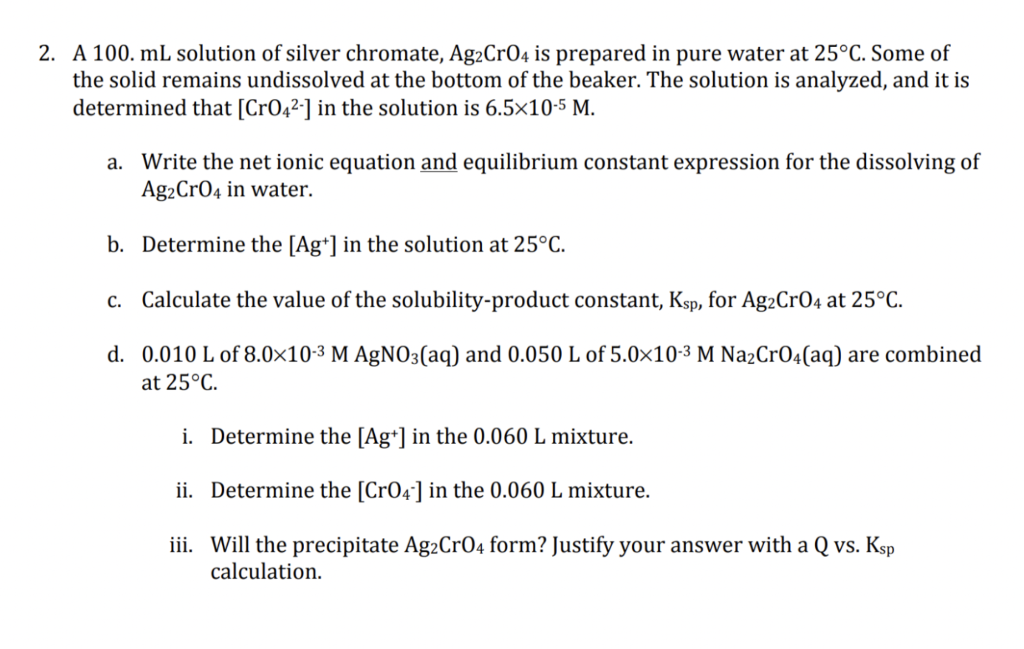
A 100. mL solution of silver chromate, Ag2CrO4 is prepared in pure water at 25C. Some of the solid remains undissolved at the bottom of the beaker. The solution is analyzed, and it is determined that [CrO42] in the solution is 6.5105M. a. Write the net ionic equation and equilibrium constant expression for the dissolving of Ag2CrO4 in water. b. Determine the [Ag+]in the solution at 25C. c. Calculate the value of the solubility-product constant, Ksp, for Ag2CrO4 at 25C. d. 0.010L of 8.0103MAgNO3(aq) and 0.050L of 5.0103MNa2CrO4(aq) are combined at 25C. i. Determine the [Ag+]in the 0.060L mixture. ii. Determine the [CrO4]in the 0.060L mixture. iii. Will the precipitate Ag2CrO4 form? Justify your answer with a Q vs. Ksp calculation
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


