Question: Hi - In two attempts I've got the answer to the question below wrong !?! - what is your expert view please ? Many Thanks
Hi -
In two attempts I've got the answer to the question below wrong !?! - what is your expert view please ?
Many Thanks
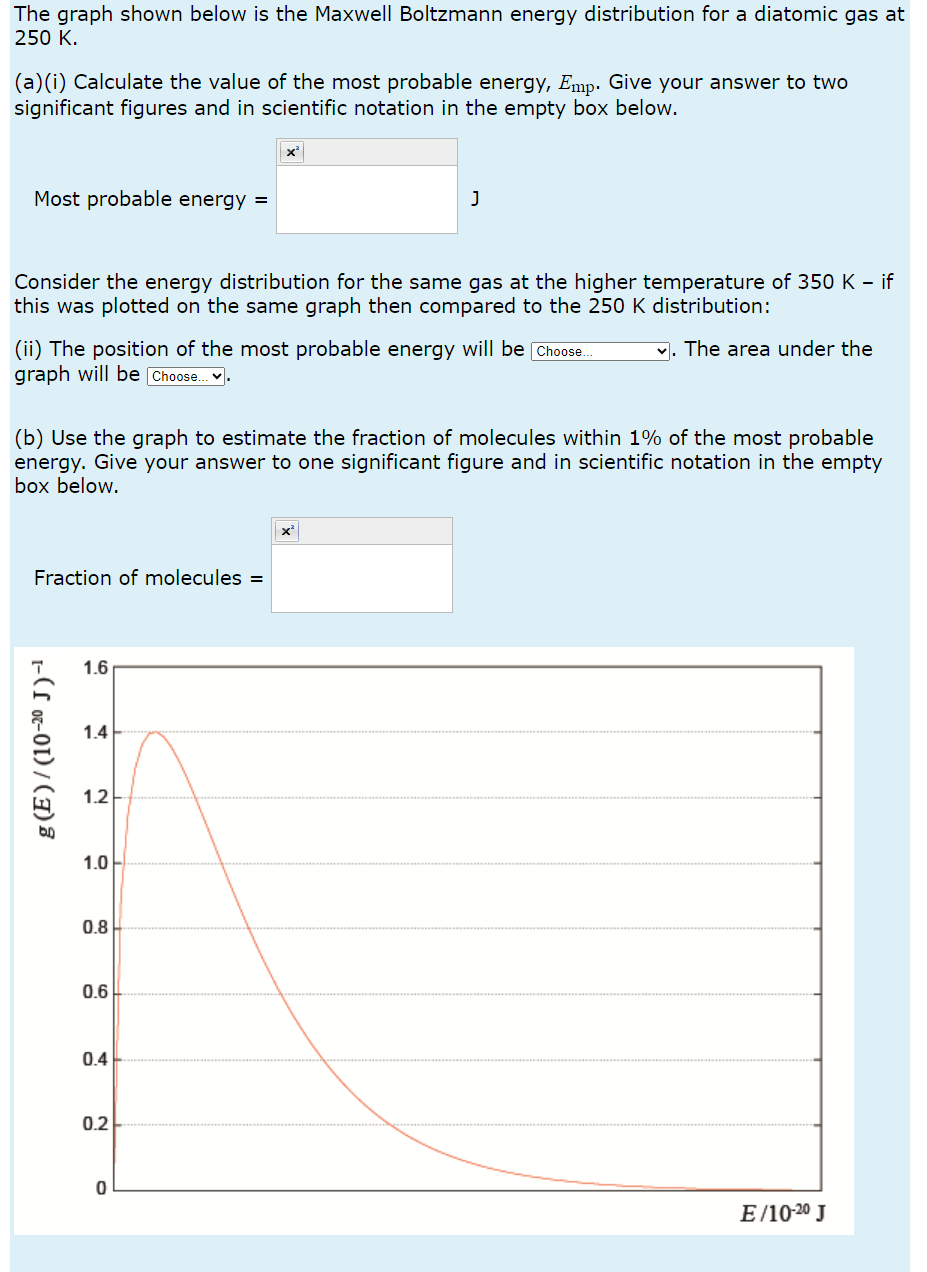
The graph shown below is the Maxwell Boltzmann energy distribution for a diatomic gas at 250 K. (a)(i) Calculate the value of the most probable energy, Emp. Give your answer to two significant figures and in scientific notation in the empty box below. x'I Most probable energy = J Consider the energy distribution for the same gas at the higher temperature of 350 K if this was plotted on the same graph then compared to the 251'.)| K distribution: (ii) The position of the most probable energy will be . The area under the graph will be _. (b) Use the graph to estimate the fraction of molecules within 1% of the most probable energy. Give your answer to one significant figure and in scientific notation in the empty box below. le Fraction of molecules = '7 1.6 -'x H g. 2 1.4r X 0"\". 1.2 a E an 1.0 4 oar- a 05* a on a 0.2 -. D EJIONJ
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


