Question: hi need some help with this question please (b) The spirocyclic pentadiene derivative F shown below is converted stereospecifically into compound G on heating. The
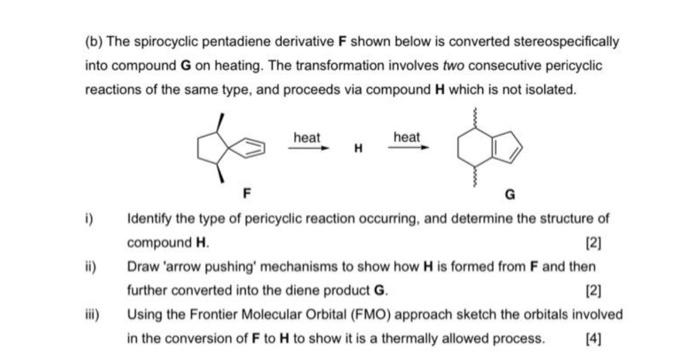
(b) The spirocyclic pentadiene derivative F shown below is converted stereospecifically into compound G on heating. The transformation involves two consecutive pericyclic reactions of the same type, and proceeds via compound H which is not isolated. i) Identify the type of pericyclic reaction occurring, and determine the structure of compound H. [2] ii) Draw 'arrow pushing' mechanisms to show how H is formed from F and then further converted into the diene product G. [2] iii) Using the Frontier Molecular Orbital (FMO) approach sketch the orbitals involved in the conversion of F to H to show it is a thermally allowed process. [4] (b) The spirocyclic pentadiene derivative F shown below is converted stereospecifically into compound G on heating. The transformation involves two consecutive pericyclic reactions of the same type, and proceeds via compound H which is not isolated. i) Identify the type of pericyclic reaction occurring, and determine the structure of compound H. [2] ii) Draw 'arrow pushing' mechanisms to show how H is formed from F and then further converted into the diene product G. [2] iii) Using the Frontier Molecular Orbital (FMO) approach sketch the orbitals involved in the conversion of F to H to show it is a thermally allowed process. [4]
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


