Question: hi please help me with this question it is not 0.0316 or 48.68 0051.0 points A 1.000L vessel is filled with 1.000 mole of N2,
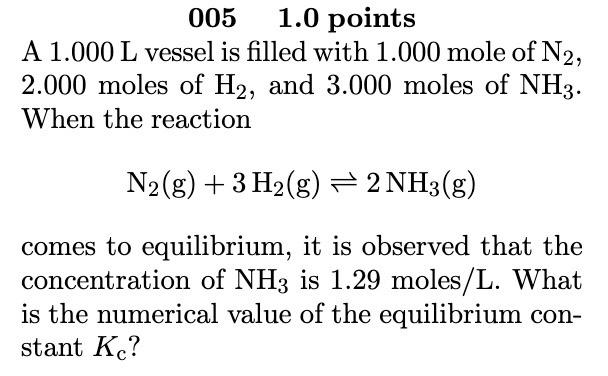
0051.0 points A 1.000L vessel is filled with 1.000 mole of N2, 2.000 moles of H2, and 3.000 moles of NH3. When the reaction N2(g)+3H2(g)2NH3(g) comes to equilibrium, it is observed that the concentration of NH3 is 1.29 moles/L. What is the numerical value of the equilibrium constant Kc
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


