Question: Hi. Would it be possible to get some help on how to get mmol HCl/mg tablet + how to do the following required questions? I'm
Hi. Would it be possible to get some help on how to get mmol HCl/mg tablet + how to do the following required questions?
I'm not sure what other numbers are needed so I added a screenshot of the other numbers I used in the previous table.
Thank you so much!
Table to be solved: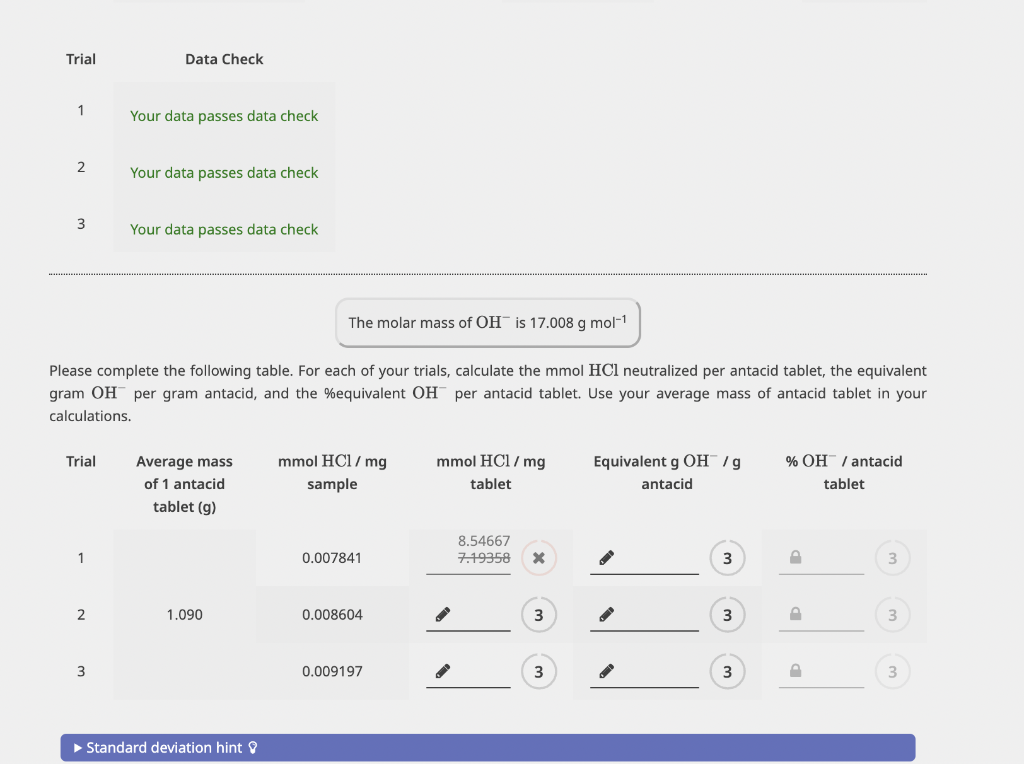
Table with Data:
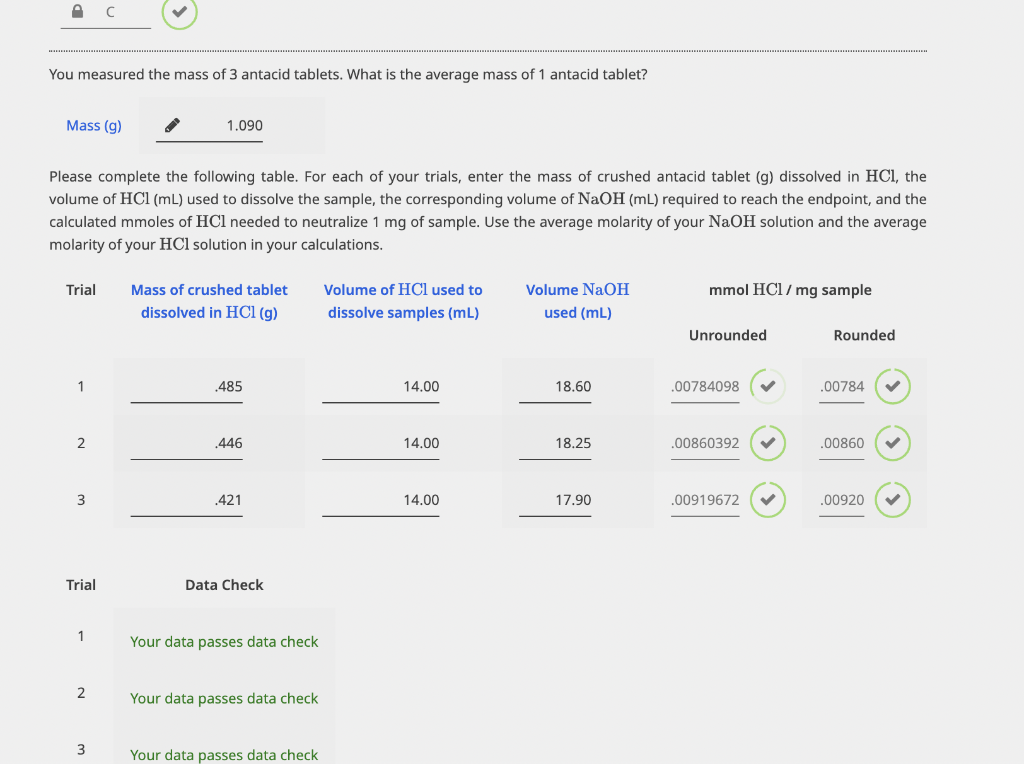
Please complete the following table. For each of your trials, calculate the mmolHCl neutralized per antacid tablet, the equivalent gram OHper gram antacid, and the \%equivalent OHper antacid tablet. Use your average mass of antacid tablet in your calculations. You measured the mass of 3 antacid tablets. What is the average mass of 1 antacid tablet? Mass (g) Please complete the following table. For each of your trials, enter the mass of crushed antacid tablet (g) dissolved in HCl, the volume of HCl(mL) used to dissolve the sample, the corresponding volume of NaOH(mL) required to reach the endpoint, and the calculated mmoles of HCl needed to neutralize 1mg of sample. Use the average molarity of your NaOH solution and the average molarity of your HCl solution in your calculations. Please complete the following table. For each of your trials, calculate the mmolHCl neutralized per antacid tablet, the equivalent gram OHper gram antacid, and the \%equivalent OHper antacid tablet. Use your average mass of antacid tablet in your calculations. You measured the mass of 3 antacid tablets. What is the average mass of 1 antacid tablet? Mass (g) Please complete the following table. For each of your trials, enter the mass of crushed antacid tablet (g) dissolved in HCl, the volume of HCl(mL) used to dissolve the sample, the corresponding volume of NaOH(mL) required to reach the endpoint, and the calculated mmoles of HCl needed to neutralize 1mg of sample. Use the average molarity of your NaOH solution and the average molarity of your HCl solution in your calculations
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


