Question: HINT Enter your answer in the provided box. A solution of 1.15g of solute dissolved in 25.0mL of H2O at 25C has a boiling point
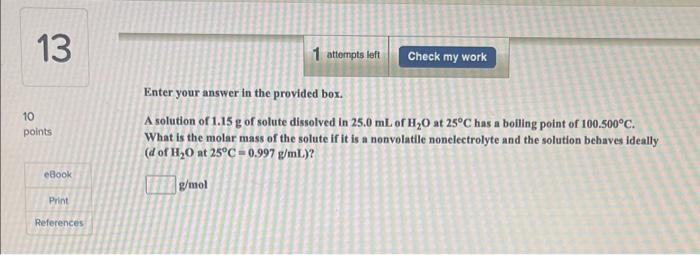
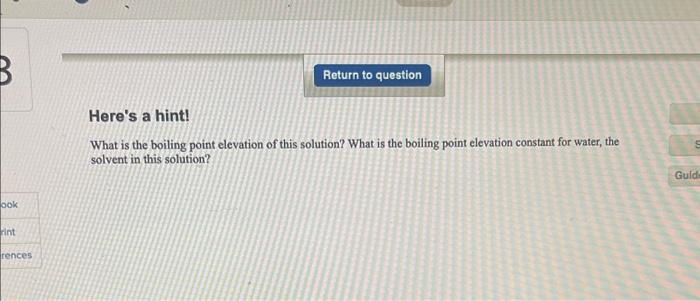
Enter your answer in the provided box. A solution of 1.15g of solute dissolved in 25.0mL of H2O at 25C has a boiling point of 100.500C. What is the molar mass of the solute if it is a nonvolatile nonelectrolyte and the solution behaves ideally (d of H2O at 25C=0.997g/mL) ? g/mol Here's a hint! What is the boiling point elevation of this solution? What is the boiling point elevation constant for water, the solyent in this solution
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


