Question: hint: start from the equilibrium constant equation and write the assumption. Using the equation of state = , where z is the compressibility factor, and
hint: start from the equilibrium constant equation and write the assumption. Using the equation of state = , where z is the compressibility factor, and definition of molar concentration to solve the part a.
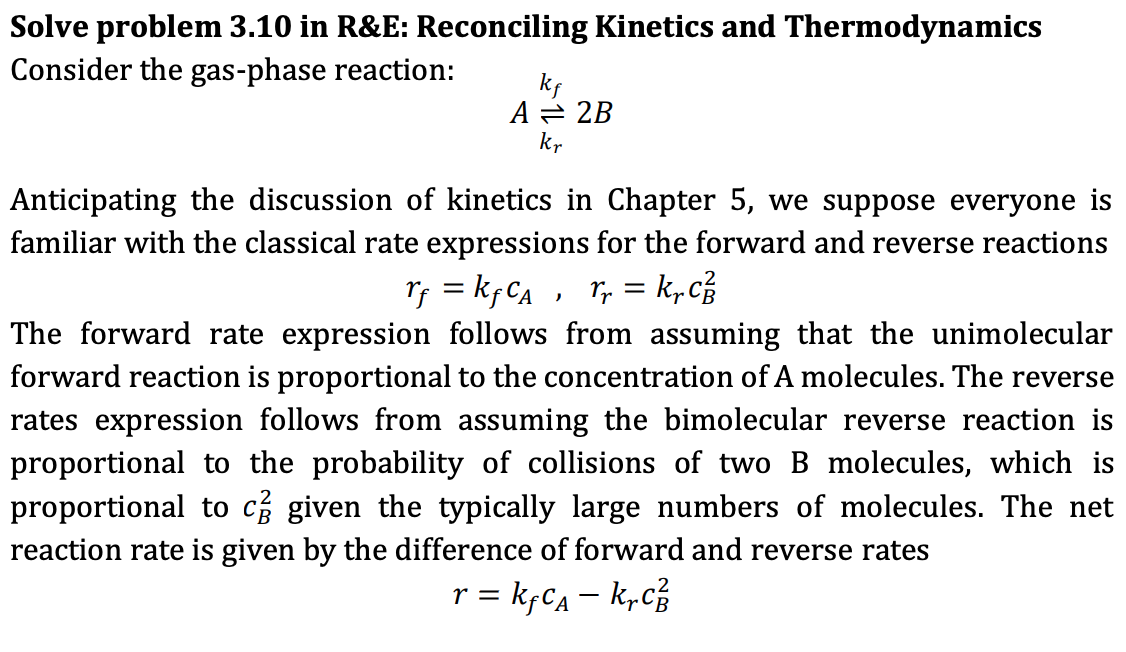
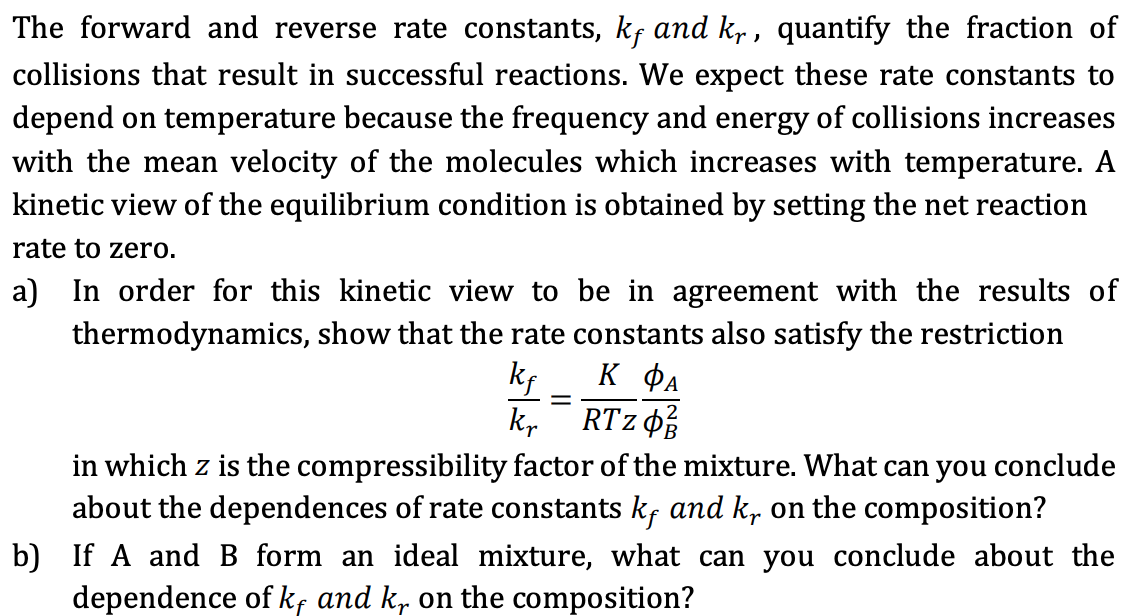
Solve problem 3.10 in R&E: Reconciling Kinetics and Thermodynamics Consider the gas-phase reaction: ke A = 2B kr Anticipating the discussion of kinetics in Chapter 5, we suppose everyone is familiar with the classical rate expressions for the forward and reverse reactions rf = kf Ca , rr = k, The forward rate expression follows from assuming that the unimolecular forward reaction is proportional to the concentration of A molecules. The reverse rates expression follows from assuming the bimolecular reverse reaction is proportional to the probability of collisions of two B molecules, which is proportional to c given the typically large numbers of molecules. The net reaction rate is given by the difference of forward and reverse rates r = kyca - krc The forward and reverse rate constants, kf and kr, quantify the fraction of collisions that result in successful reactions. We expect these rate constants to depend on temperature because the frequency and energy of collisions increases with the mean velocity of the molecules which increases with temperature. A kinetic view of the equilibrium condition is obtained by setting the net reaction rate to zero. a) In order for this kinetic view to be in agreement with the results of thermodynamics, show that the rate constants also satisfy the restriction ki kr in which z is the compressibility factor of the mixture. What can you conclude about the dependences of rate constants ky and ky on the composition? b) If A and B form an ideal mixture, what can you conclude about the dependence of kf and kr on the composition? = RTZ OB 2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


