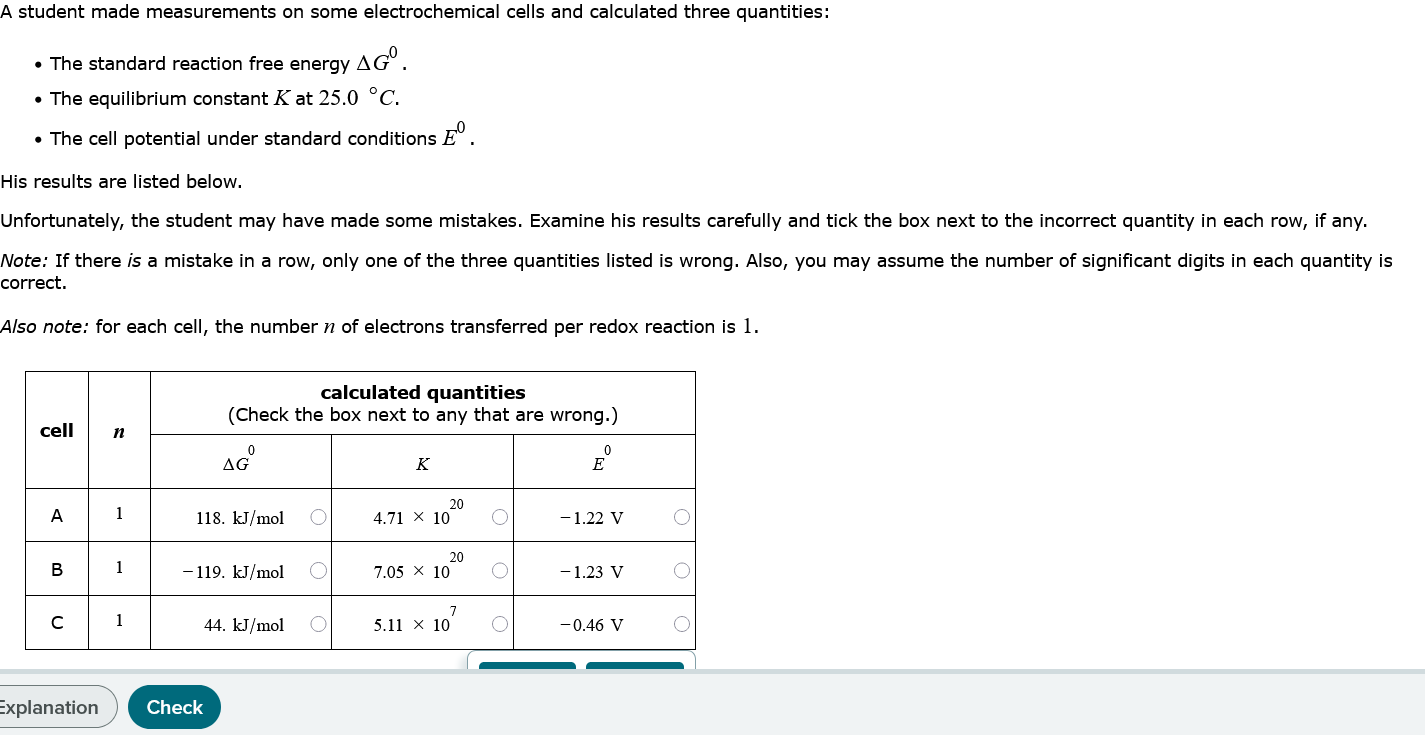
Question: How about this one? Please find the incorrect value for each cell one at a time. A student made measurements on some electrochemical cells and
How about this one? Please find the incorrect value for each cell one at a time.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


