Question: How can I solve this using NON-LINEAR LEAST SQUARE FITTING for both of these series? I understand how to solve for the rydberg constant by
How can I solve this using NON-LINEAR LEAST SQUARE FITTING for both of these series? I understand how to solve for the rydberg constant by simultaneous nonlinear equations please DO NOT answer that part of the question. I only need to understand how to solve using non-linear least square fitting.

Here is my professors solution -- but I don't know how to use solver in excel, unless someone can help explain how to use it -- is there another method to solving this besides solver for non-linear least square fitting?
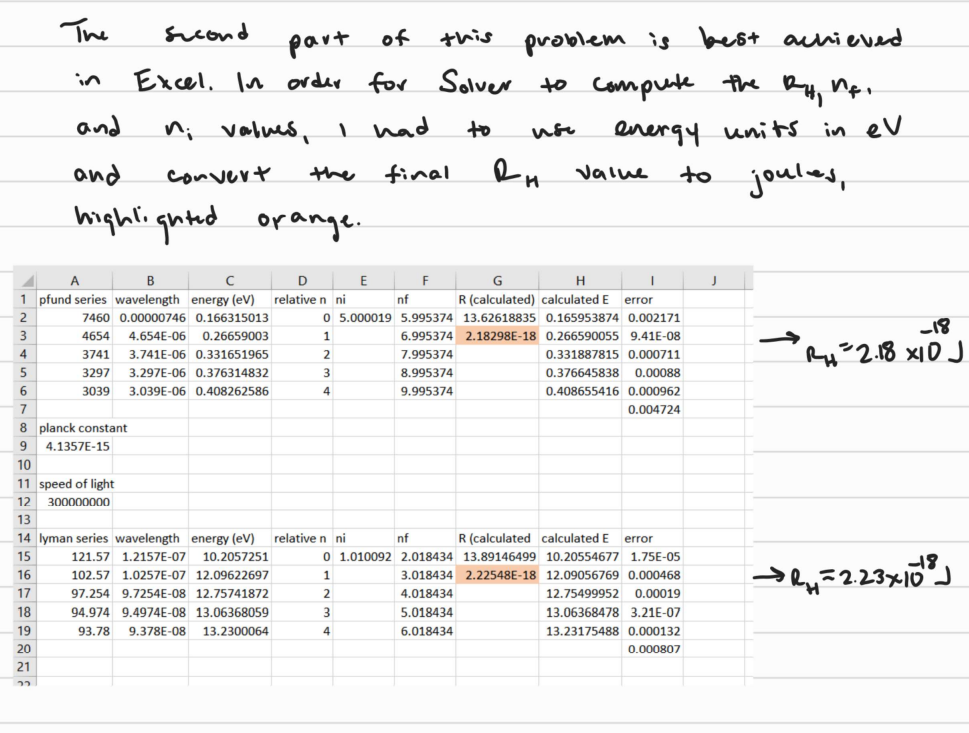
11) The Pfund series in the Hydrogen line spectrum has the wavelengths: (in nm) 74604654374132973039 The Lyman series has the wavelengths: (in nm ) 121.57102.5797.25494.97493.780 Pick any series of three wavelengths in the Pfund series and determine the Rydberg constant by solving simultaneous nonlinear equations. Do the same for the Lyman series. Next, use non-linear least-squares fitting to determine the Rydberg constant using all of the wavelengths in the Pfund series. Do the same for the Lyman series. Finally, look up the accepted value of the Rydberg constant and determine which approach gives the Rydberg constant in the best agreement with the accepted value. The sucond part of this problem is best achieved in Excel. In order for Solver to compute the RH1nf. and ni values, 1 had to use energy units in eV and convert the final RH value to joules, highlignted orange
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


