Question: how can you do the following: a) b) Dissolve 219.5 mg anhydrous KH2PO4 in distilled water and dilute to 1000 ml; (record the actual amount
how can you do the following:
a)
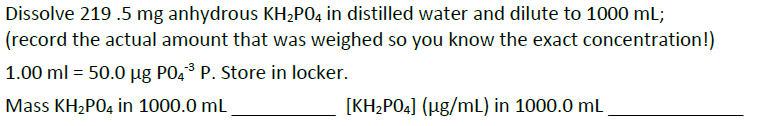
b)

Dissolve 219.5 mg anhydrous KH2PO4 in distilled water and dilute to 1000 ml; (record the actual amount that was weighed so you know the exact concentration!) 1.00 ml = 50.0 ug P04 P. Store in locker. Mass KH2P04 in 1000.0 mL [KH2PO4] (ug/mL) in 1000.0 mL Phosphate standards will be prepared in the range from (ie.) 0.200,0.500, 1.00, 2.00, 3.00 and 4.00 pg/mL in dH2O using the stock KH2PO4 solution (50.00 pg/ml) in 50.00 mL volumetric flasks using volumetric syringes and pipettes ranging in volume from approx. 0.200 to 4.00 ml. *note: this range is a suggestion. Anything from 0.2 to 5.0 will work, as long as all 6 standards are distributed somewhat evenly between 0.2 and 5 pg/mL
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


