Question: How could you isolate para-nitro benzoic acid 2 after alkaline hydrolysis of ethyl 4nitrobenzoate 1 ? Note that the product of the reaction is ethyl
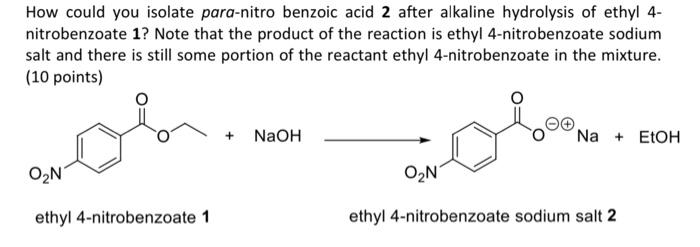
How could you isolate para-nitro benzoic acid 2 after alkaline hydrolysis of ethyl 4nitrobenzoate 1 ? Note that the product of the reaction is ethyl 4-nitrobenzoate sodium salt and there is still some portion of the reactant ethyl 4-nitrobenzoate in the mixture. (10 points) ethyl 4-nitrobenzoate 1 ethyl 4-nitrobenzoate sodium salt 2
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


