Question: How do I do part b? - You are tasked by a gem store to calculate the density of a diamond. You do four replicate
 How do I do part b?
How do I do part b?
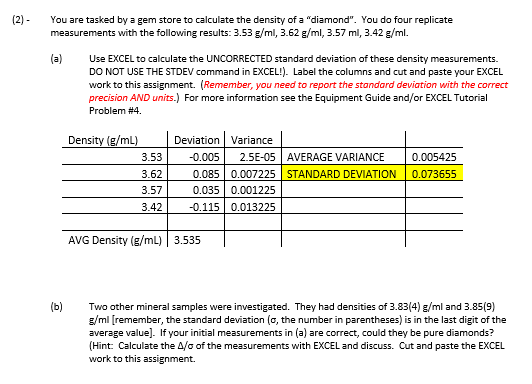
- You are tasked by a gem store to calculate the density of a "diamond". You do four replicate measurements with the following results: 3.53g/ml,3.62g/ml,3.57ml,3.42g/ml. (a) Use EXCEL to calculate the UNCORRECTED standard deviation of these density measurements. DO NOT USE THE STDEV command in EXCEL!). Label the columns and cut and paste your EXCEL work to this assignment. (Remember, you need to report the standard deviation with the correct precision AND units.) For more information see the Equipment Guide and/or EXCEL Tutorial Problem #4. (b) Two other mineral samples were investigated. They had densities of 3.83(4)g/ml and 3.85(9) g/ml [remember, the standard deviation ( , the number in parentheses) is in the last digit of the average value]. If your initial measurements in (a) are correct, could they be pure diamonds? (Hint Calculate the / of the measurements with EXCEL and discuss. Cut and paste the EXCEL work to this assignment
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


