Question: How do I do this? The following data were obtained for the reaction given below. 2ClO2(aq)+2OH(aq)ClO3(aq)+ClO2(aq)+H2O(l)Rate=t[ClO2] (a) Determine the rate law and the value of
How do I do this? 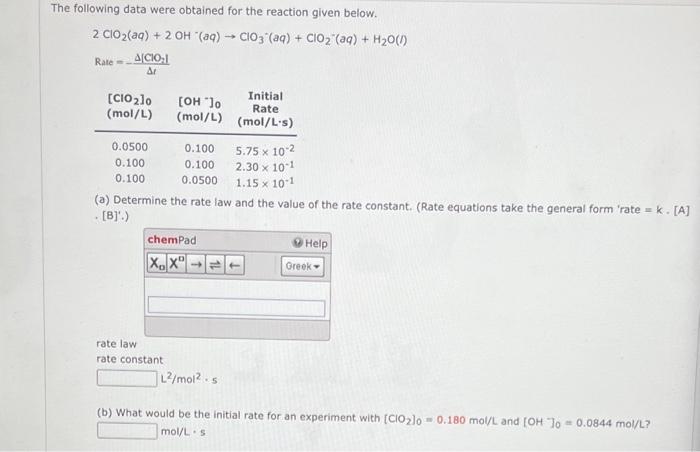

The following data were obtained for the reaction given below. 2ClO2(aq)+2OH(aq)ClO3(aq)+ClO2(aq)+H2O(l)Rate=t[ClO2] (a) Determine the rate law and the value of the rate constant. (Rate equations take the general form 'rate = k. [A] [B].) rate law rate constant L2/mol25 (b) What would be the initial rate for an experiment with [ClO2]0=0.180mol/L and [OH]0=0.0844mol/L ? mol/Ls
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


