Question: Homework!!! Help Short Background Information and Formulas In this experiment, the kinetics of a clock reaction between iodide ions (1) and peroxydisulfate ions (S2O82-) was


Homework!!! Help
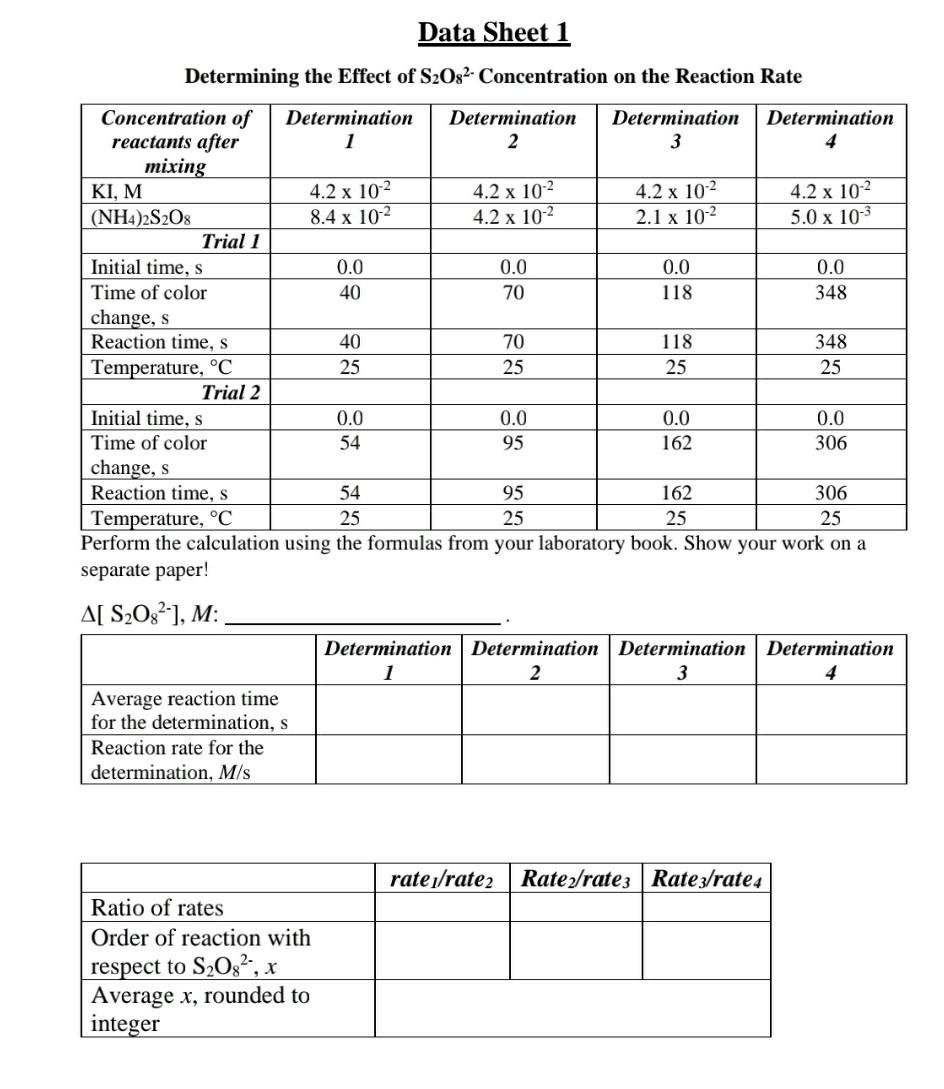
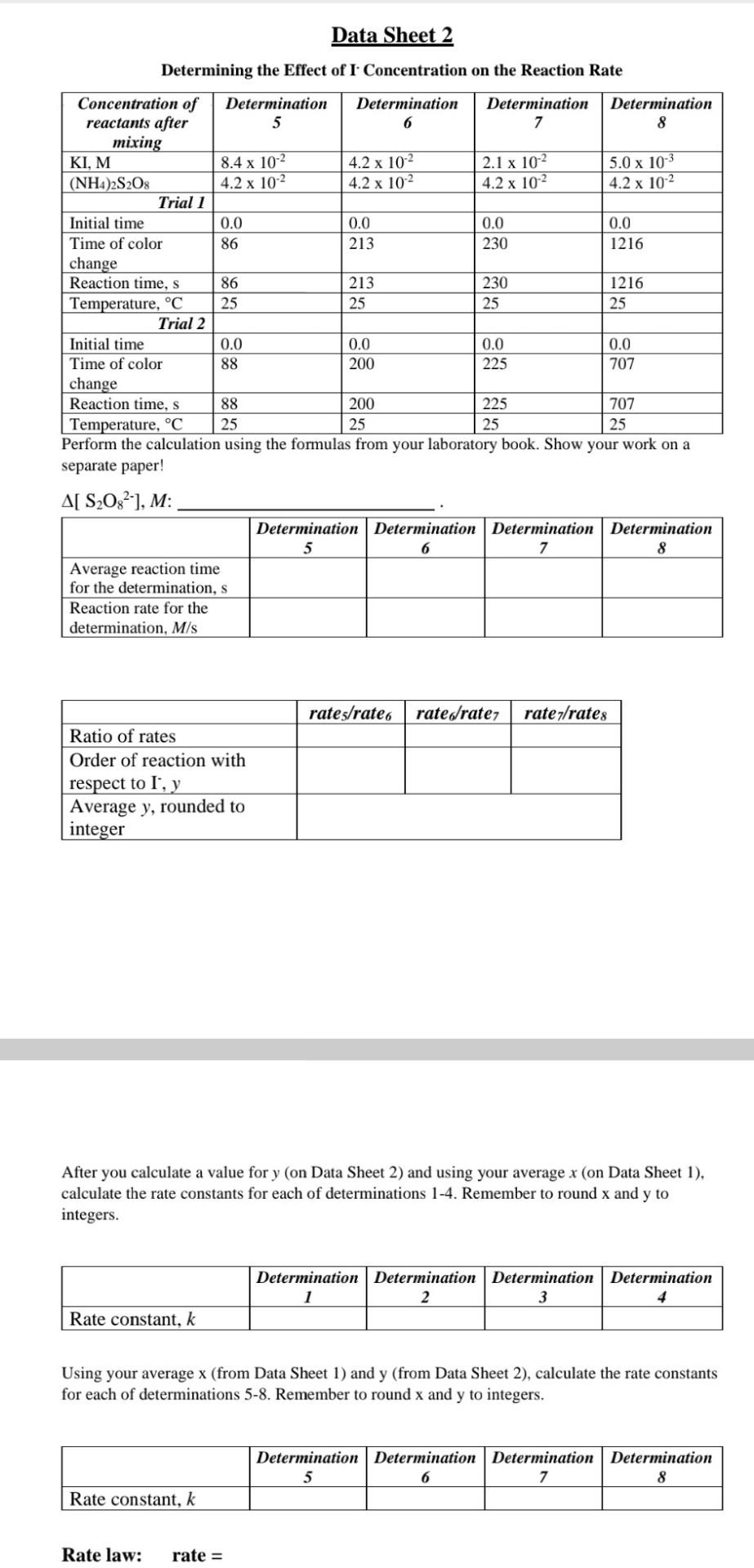
Short Background Information and Formulas In this experiment, the kinetics of a clock reaction between iodide ions (1) and peroxydisulfate ions (S2O82-) was studied. The reaction shown below: S208 2 (aq) + 31(aq) 25042 aq) + 13 (aq) During the reaction, ammonium peroxodisulfate, (NH4)2S2O3, and potassium iodide (KI), solutions supplied these ions. The rate law for this relatively slow reaction is Rate = k[S2082-'[ 25203 2 (aq) + 13 (aq) Furthermore, starch solution and a constant amount of sodium thiosulfate (Na2S203) was added to each reaction mixture. Starch forms a deep blue complex with triiodide ions (13'). The thiosulfate (S2032-) react rapidly with the iodide (I), according to the reaction below: 7 S4062 (aq) + 31 (aq) As soon as all the thiosulfate (S2032) has reacted, triiodide (13') can accumulate and its presence will be indicated by the dark blue color of the starch-Iz complex in the solution. Hence, the thiosulfate (S2032) acts as a built-in clock indicating when different reaction mixtures have reacted to the same point of completion. You will use the method of initial rate described by your laboratory book to study the S208?- I reaction. The reaction rate for the S2082 reaction is Rate = -(A[ S2082-)/ At) Be familiar with the rate law (rate = k[A]"[B]'[C]), reaction rate (rate = -(A[A] / At)), activation energy (Ea), Arrhenius equation (k = Ae-Ea/RT), and catalyst. For further details on the experiment, important definition, and formulas, please read the laboratory book and/or chapter 14 in your lecture book. In this experiment, you will determine the effect of concentration on the reaction rate by changing the concentration of the reactant to be studied, while all other conditions are held constant. On Data Sheet 1, you are provided with the data of a series of four reactions of S2082- and I' in which the [I] is kept constant and the [S208?) is varied. On Data Sheet 2, you are provided with the data of a series of four reactions of S2082- and I in which the [S208-) is kept constant and the is [1 ] varied. In the Video, provided with this lab assignment, you can observe the effect of temperature on the reaction rate. Use the Video to answer the Additional Questions. Data Sheet 1 Determining the Effect of S2O32- Concentration on the Reaction Rate Concentration of Determination Determination Determination Determination reactants after 1 2 3 4 mixing KI, M 4.2 x 10-2 4.2 x 102 4.2 x 10-2 4.2 x 102 (NH4)2S208 8.4 x 102 4.2 x 10-2 2.1 x 102 5.0 x 103 Trial 1 Initial time, s 0.0 0.0 0.0 0.0 Time of color 40 70 118 348 change, s Reaction time, s 40 70 118 348 Temperature, C 25 25 25 25 Trial 2 Initial time, s 0.0 0.0 0.0 0.0 Time of color 54 95 162 306 change, s Reaction time, s 54 95 162 306 Temperature, C 25 25 25 25 Perform the calculation using the formulas from your laboratory book. Show your work on a separate paper! A[ S2O8], M: Determination Determination Determination Determination 1 2 3 4 Average reaction time for the determination, s Reaction rate for the determination, M/s rate1/rate2 Rate/rates Ratez/rate 4 Ratio of rates Order of reaction with respect to S2082, x Average x, rounded to integer 0.0 Data Sheet 2 Determining the Effect of I Concentration on the Reaction Rate Concentration of Determination Determination Determination Determination reactants after 5 6 7 8 mixing KI, M 8.4 x 10-2 4.2 x 10-2 2.1 x 102 5.0 x 10-3 (NH4)2S208 4.2 x 102 4.2 x 102 4.2 x 102 4.2 x 10-2 Trial 1 Initial time 0.0 0.0 0.0 Time of color 86 213 230 1216 change Reaction time, s 86 213 230 1216 Temperature, C 25 25 25 25 Trial 2 Initial time 0.0 0.0 0.0 0.0 Time of color 88 200 225 707 change Reaction time, s 88 200 225 707 Temperature, C 25 25 25 25 Perform the calculation using the formulas from your laboratory book. Show your work on a separate paper! A[ S2O32-1, M: Determination Determination Determination Determination 5 6 7 8 Average reaction time for the determination, s Reaction rate for the determination, M/s rates/rate 6 rated/rate; rate 7/rates Ratio of rates Order of reaction with respect to l', y Average y, rounded to integer After you calculate a value for y (on Data Sheet 2) and using your average x (on Data Sheet 1), calculate the rate constants for each of determinations 1-4. Remember to round x and y to integers. Determination Determination Determination Determination 2 3 Rate constant, k Using your average x (from Data Sheet 1) and y (from Data Sheet 2), calculate the rate constants for each of determinations 5-8. Remember to round x and y to integers. Determination Determination Determination Determination 5 6 7 8 Rate constant, k Rate law: rate = Additional Questions 1. The rate is dependent on temperature. What kind of temperatures were used to study the effect of temperature on the reaction rate in the Video? Convert the Kelvin to C. 2. What did they put the beaker holding the reaction mixture into to perform the low temperature and the high temperature determinations? 3. What can you tell about the effect of temperature on the reaction rate at high temperature and at low temperature based what you saw in the Video? 4. Write down the Arrhenius equation and the linear form of the Arrhenius equation. What value was calculated in the Video by using them
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


