Question: How do I find the molarity?? begin{tabular}{|l|l|l|l|l|} hline Data & & & hline Stock Iron (mL) & Stock Thiocyanate (mL) & Water (mL) &
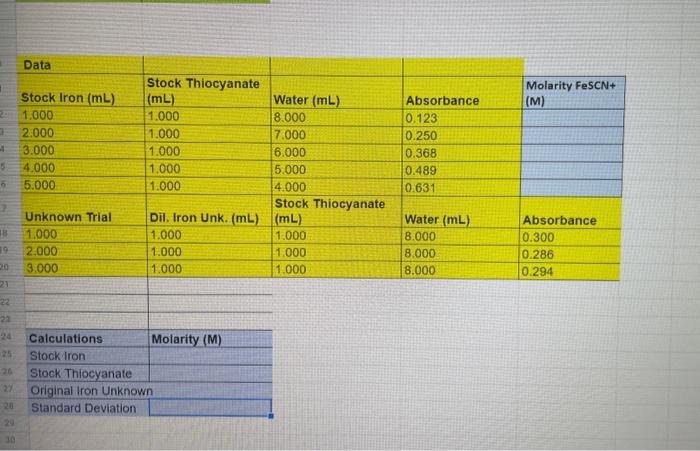
\begin{tabular}{|l|l|l|l|l|} \hline Data & & & \\ \hline Stock Iron (mL) & Stock Thiocyanate (mL) & Water (mL) & Absorbance & Molarity FeSCN+ (M) \\ \hline 1.000 & 1.000 & 8.000 & 0.123 \\ \hline 2.000 & 1.000 & 7.000 & 0.250 \\ \hline 3.000 & 1.000 & 6.000 & 0.368 \\ \hline 4.000 & 1.000 & 5.000 & 0.489 \\ \hline 5.000 & 1.000 & 4.000 & 0.631 & \\ \hline Unknown Trial & Dil. Iron Unk. (mL) & Stock Thiocyanate (mL) & Water (mL) & Absorbance \\ \hline 1.000 & 1.000 & 1.000 & 8.000 & 0.300 \\ \hline 2.000 & 1.000 & 1.000 & 8.000 & 0.286 \\ \hline 3.000 & 1.000 & 1.000 & 8.000 & 0.294 \\ \hline \end{tabular} \begin{tabular}{|l|l|} \hline Calculations & Molarity (M) \\ \hline Stock Iron & \\ \hline Stock Thiocyanate \\ \hline Original Iron Unknown \\ \hline Standard Deviation \\ \hline \end{tabular}
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


