Question: I need help with question 4. 3. (9 pts. Using the data for the 10 solutions of Cu2+, prepare a graph of Absorbance vs. Concentration

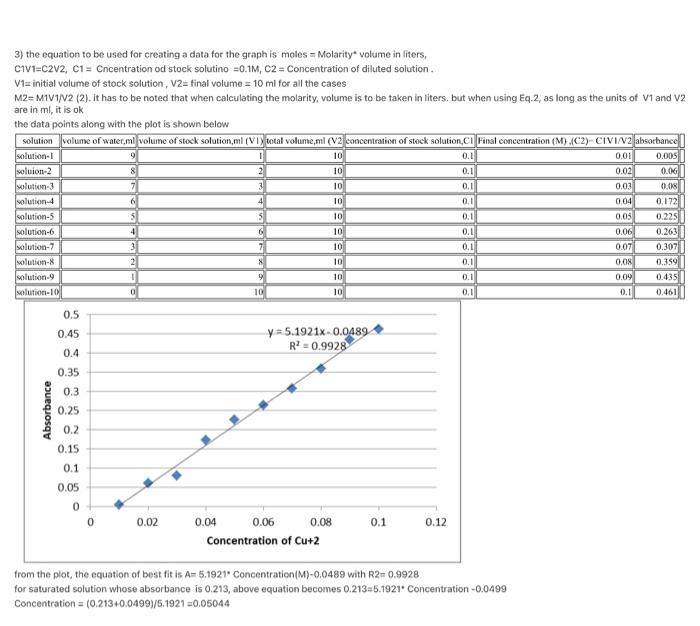
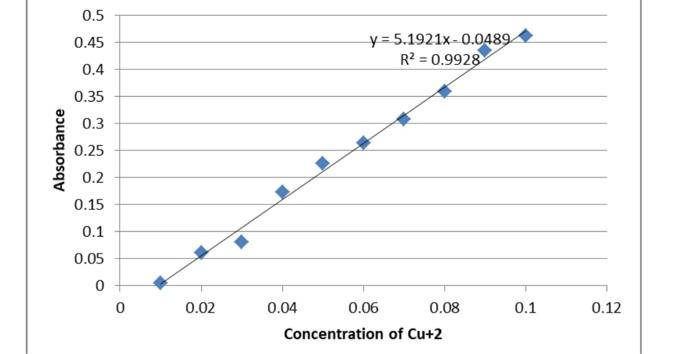
3. (9 pts. Using the data for the 10 solutions of Cu2+, prepare a graph of Absorbance vs. Concentration using a graphing program. Draw a best-fit line. Your graph should be a full page with a number, title, labeled axes including appropriate units, and display the trendline equation. ATTACH YOUR GRAPH TO THIS LAB REPORT. 4. (6 pts.) Determine the concentration of the saturated solution from Part B by using the measured absorbance value and the trendline equation. Record your result in the Result Table. 3) the equation to be used for creating a data for the graph is moles = Molarity volume in liters, CIV1=C2V2, C1 = Cncentration od stock solutino =0.1M, C2 = Concentration of diluted solution V1= initial volume of stock solution, V2= final volume = 10 ml for all the cases M2=MIV1/V2 (2). It has to be noted that when calculating the molarity, volume is to be taken in liters, but when using Eq.2, as long as the units of V1 and V2 are in ml, it is ok the data points along with the plot is shown below solution volume of water, mi volume of stock solution, ml (VI) wotal volume, mil (v2 concentration of stock solution,Ci Final concentration (M) (C2) CIVI/2 absorbance solution soluion-2 0.01 9 8 1 2 3 10 10 0.1 0.1 0.02 0.03 0.00 0.06 0.08 10 10 6 4 5 0.1 0.1 0.1 0.1 10 6 solution 3 solution 4 solution-5 solution-6 solution-7 solution- solution.9 solution-101 10 10 10 0.04 0.05 0.06 0.07 0.08 0.1 0.1 0.1 0.172 0.225 0.263 0.307 0.359 0.435 0.461 9 0.09 10 10 0 10 0.1 0.1 0,5 0.45 0.4 y=5.1921x. 0.0489 R2 = 0.9928 0.35 0.3 Absorbance 0.25 0.2 0.15 0.1 0.05 0 0 0.02 0.1 0.12 0.04 0.06 0.08 Concentration of Cu+2 from the plot, the equation of best fit is A-5.1921. Concentration(M)-0.0489 with R2=0.9928 for saturated solution whose absorbance is 0.213, above equation becomes 0.213=5.1921. Concentration-0.0499 Concentration = (0.213+0.0499/5.1921 +0.05044 0.5 0.45 0.4 y=5.1921x-0.0489 R2 = 0.9928 0.35 0.3 Absorbance 0.25 0.2 0.15 0.1 0.05 0 0 0.02 0.04 0.06 0.08 0.1 0.12 Concentration of Cu+2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


