Question: how do i get the graph to look like this? Real Gasses Ammonia vapor is compressed to 60 bar and 105C. Use the SRK equation

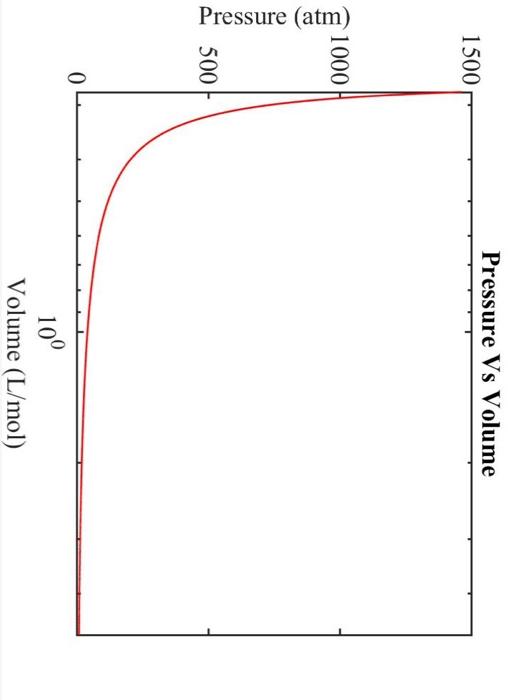
Real Gasses Ammonia vapor is compressed to 60 bar and 105C. Use the SRK equation of state to determine the vapor mass density (g/L). For ammonia, Tc=405.5K,Pc=111.3atm, and =0.257. a. Determine Tr,a,b,m, and . b. Plot the pressure as a function of specific volume over a range of 0.055L/mol. Make sure to properly label the axes. Plot a sufficient number of points to get a smooth curve and use a logarithmic scale for the x-axis. You must use Excel to prepare your plot. Axes should be properly labelled, and the plot must have a title. c. Determine the specific volume of ammonia vapor at 60 bar and 105C(L/mol). d. Determine the vapor mass density (g/L)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


