Question: How do I know which pKa value I should use. For this exercise it looks like the correct one was 2.15, but I don't know
How do I know which pKa value I should use. For this exercise it looks like the correct one was 2.15, but I don't know why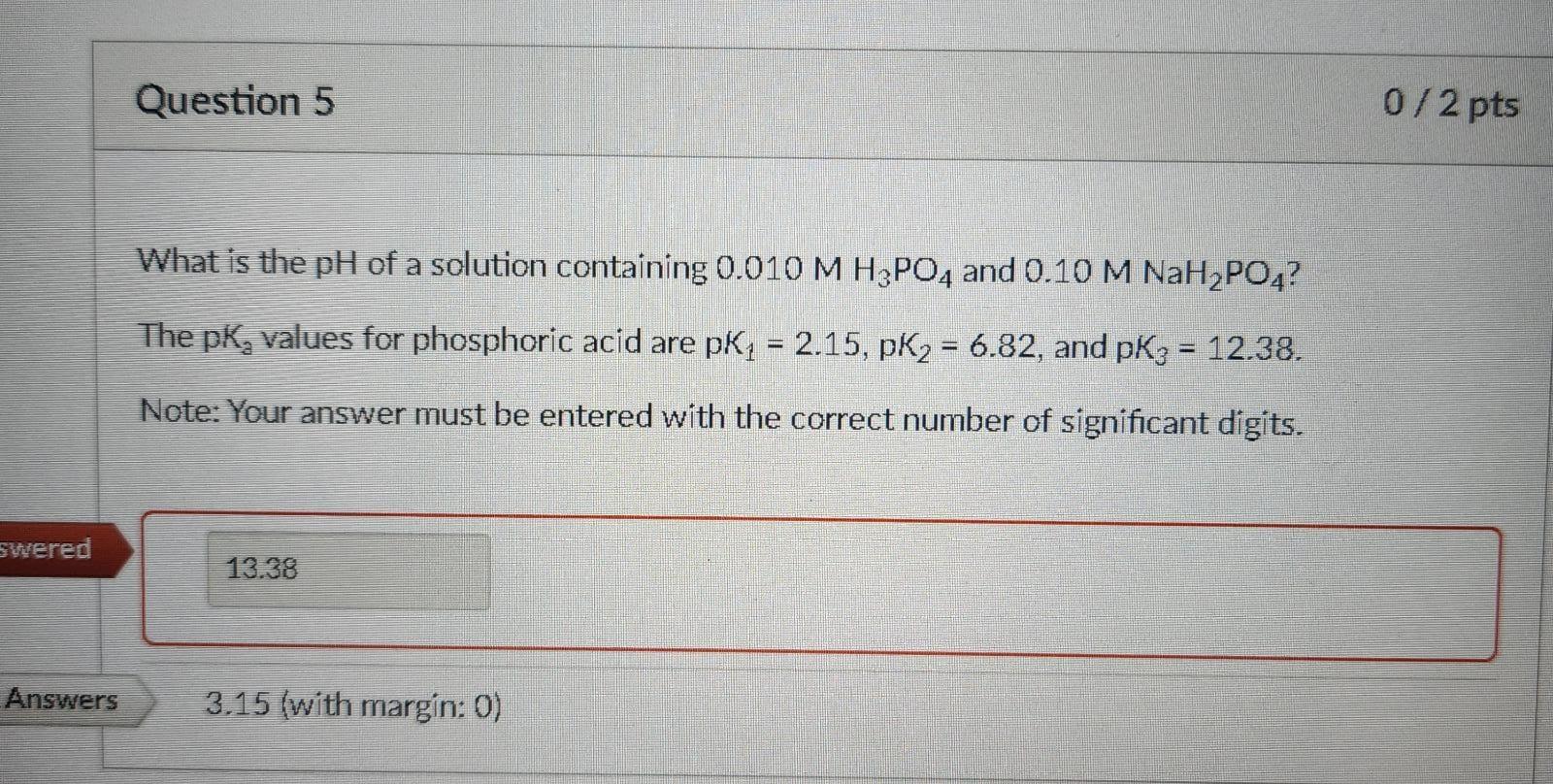
What is the pH of a solution containing 0.010MH3PO4 and 0.10MNaHPO4 ? The pK2 values for phosphoric acid are pK1=2.15,pK2=6.82, and pK3=12.38. Note: Your answer must be entered with the correct number of significant digits. 3.15 (with margin: 0)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


