Question: I am having trouble trying to answer this question the assigned amino acid is Histidine Instructions: Complete the following problems and be sure to show
I am having trouble trying to answer this question the assigned amino acid is Histidine

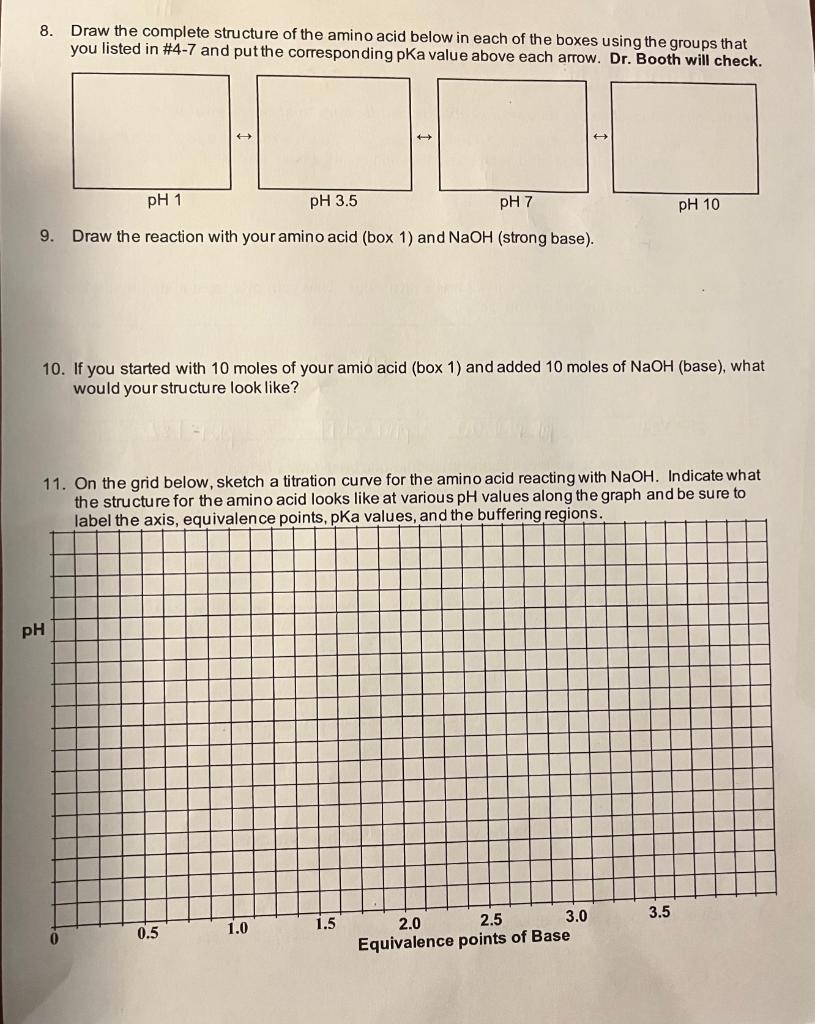
Instructions: Complete the following problems and be sure to show all your work. 1. Draw your group's amino acid below. Consider how you drew it and circle each group that can act as an acid and label it "acid" and each group that can act as a base and label it "base". HN HND 2. Each of the groups that you circled in #2 has a pka value. Use your pka table to determine the pka values for each group that you circled. Group NH3 pka Value pkg= 6.00 9.17 pka pkg= 1.82 3. For each group above, draw the acid and the conjugate base forms below. Group 1 Acid/Conj Base Group 2 Acid/Conj Base Group 3 Acid/Conj Base 4. For each of the groups above, draw the one that you would have the most of at pH 1. 5. For each of the groups above, draw the one that you would have the most of at pH 3.5. 6. For each of the groups above, draw the one that you would have the most of at pH 7. 7. For each of the groups above, draw the one that you would have the most of at pH 10. 8. Draw the complete structure of the amino acid below in each of the boxes using the groups that you listed in #4-7 and put the corresponding pka value above each arrow. Dr. Booth will check. pH 1 pH 3.5 pH 7 pH 10 9. Draw the reaction with your amino acid (box 1) and NaOH (strong base). 10. If you started with 10 moles of your amio acid (box 1) and added 10 moles of NaOH (base), what would your structure look like? 11. On the grid below, sketch a titration curve for the amino acid reacting with NaOH. Indicate what the structure for the amino acid looks like at various pH values along the graph and be sure to label the axis, equivalence points, pka values, and the buffering regions. PH 3.5 1.5 1.0 0 0.5 2.0 2.5 3.0 Equivalence points of Base
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


