Question: How do i solve for the rate laws in table IC.6 please solve? Complete the following table by calculating the initial concentration of reagents. Be
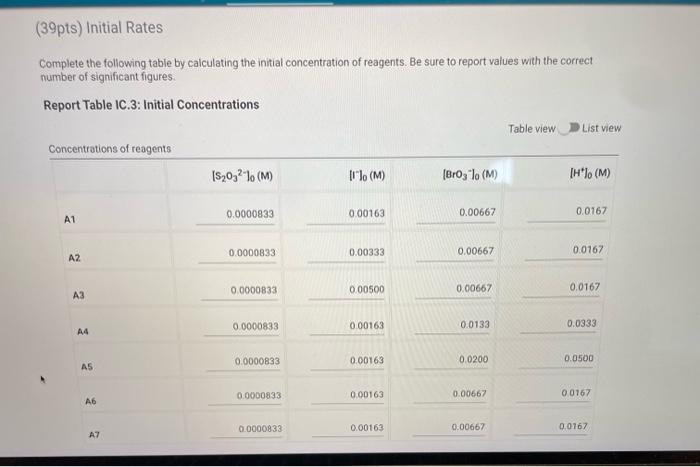
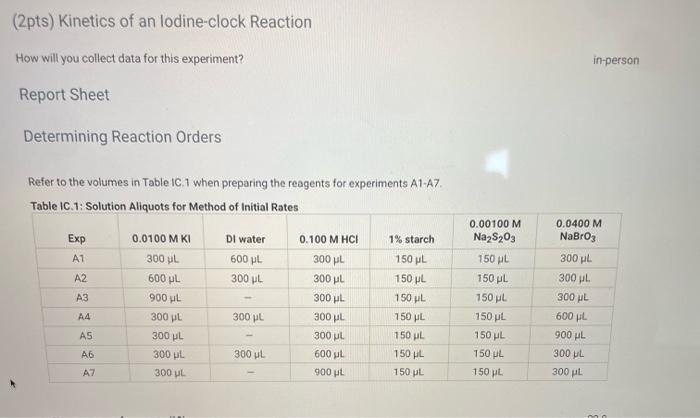
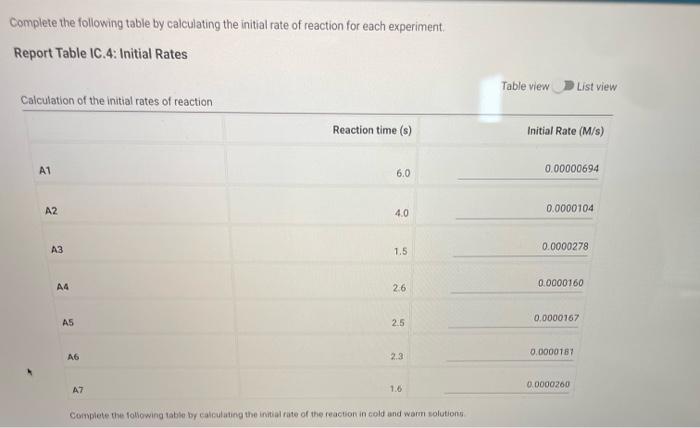
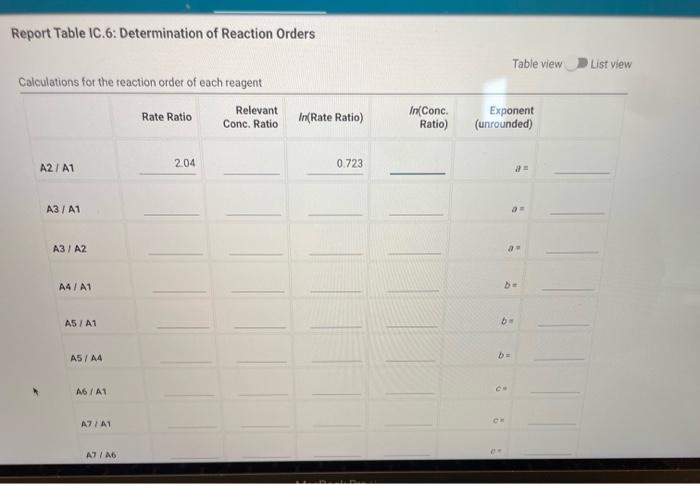
Complete the following table by calculating the initial concentration of reagents. Be sure to report values with the correct number of significant figures. Report Table IC.3: Initial Concentrations Table view List view (2pts) Kinetics of an lodine-clock Reaction How will you collect data for this experiment? in-person Report Sheet Determining Reaction Orders Refer to the volumes in Table IC. 1 when preparing the reagents for experiments A1-A7. Table IC.1: Solution Aliquots for Method of Initial Rates \begin{tabular}{|c|c|c|c|c|c|c|} \hline Exp & 0.0100MKI & DI water & 0.100MHCl & 1% starch & 0.00100MNa2S2O3 & 0.0400MNaBrO \\ \hline A1 & 300L & 600L & 300L & 150L & 150L & 300L \\ \hline A2 & 600L & 300L & 300L & 150L & 150L & 300L \\ \hline A3 & 900L & & 300L & 150L & 150L & 300L \\ \hline A4 & 300L & 300L & 300L & 150L & 150L & 600L \\ \hline A5 & 300L & & 300L & 150L & 150L & 900L \\ \hline A6 & 300L & 300L & 600L & 150L & 150L & 300L \\ \hline A7 & 300L & & 900L & 150L & 150L & 300L \\ \hline \end{tabular} Complete the following table by calculating the initial rate of reaction for each experiment. Report Table IC.4: Initial Rates Cornplete the following table by calculatieg the initial rate of the reaction in cold and warm solutions Report Table IC.6: Determination of Reaction Orders Table view List view
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


