Question: How do I solve this thermal physics problem? Question: 0.0777 mols of an ideal gas is slowly compressed in a thermally conducting cylinder from 1.32
How do I solve this thermal physics problem?
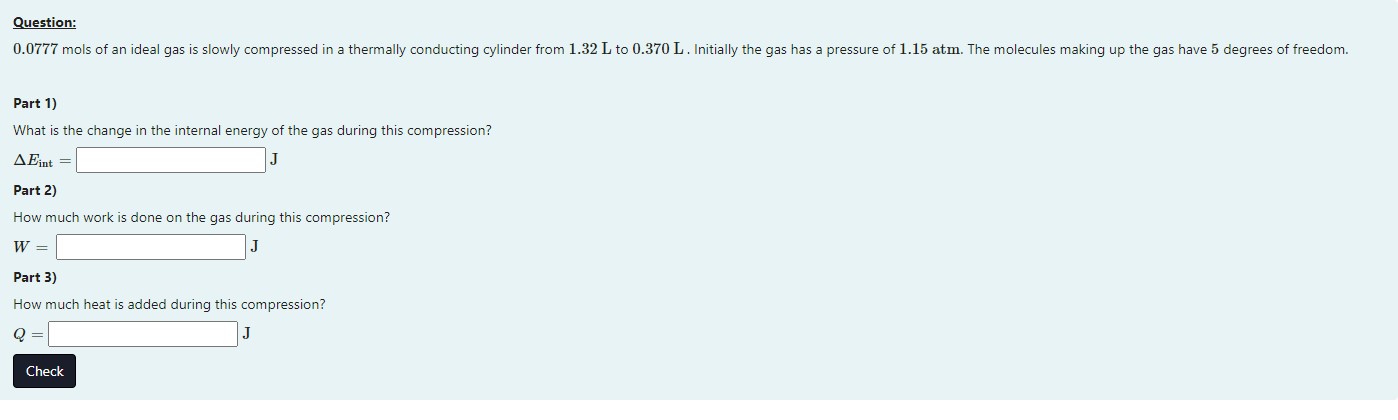
Question: 0.0777 mols of an ideal gas is slowly compressed in a thermally conducting cylinder from 1.32 L to 0.370 L . Initially the gas has a pressure of 1.15 atm. The molecules making up the gas have 5 degrees of freedom. Part 1) What is the change in the internal energy of the gas during this compression? A Eint = Part 2) How much work is done on the gas during this compression? W = Part 3) How much heat is added during this compression? Q = Check
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


