Question: how do you find the concentration (assuming that the initial concentration of Penicilin is 0.1 M)? Penicillin G reacts with hydroxylamine (NO2OH) according to the
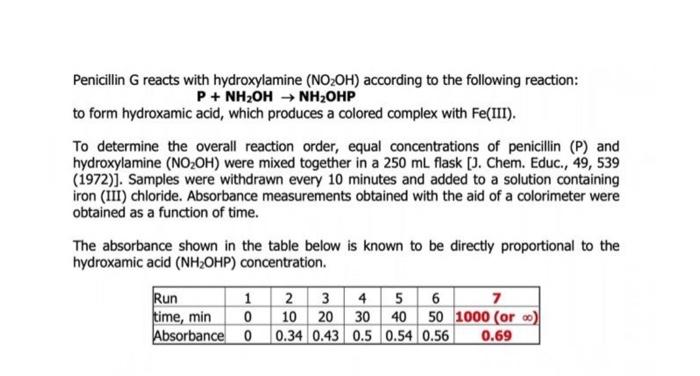
Penicillin G reacts with hydroxylamine (NO2OH) according to the following reaction: P + NH2OH NH2OHP to form hydroxamic acid, which produces a colored complex with Fe(III). To determine the overall reaction order, equal concentrations of penicillin (P) and hydroxylamine (NO2OH) were mixed together in a 250 ml flask [J. Chem. Educ., 49, 539 (1972)). Samples were withdrawn every 10 minutes and added to a solution containing iron (III) chloride. Absorbance measurements obtained with the aid of a colorimeter were obtained as a function of time. The absorbance shown in the table below is known to be directly proportional to the hydroxamic acid (NH2OHP) concentration. Run 1 2 3 4 5 6 7 time, min 0 10 20 30 40 50 1000 (oro) Absorbance 0 0.34 0.43 0.5 0.54 0.56 0.69
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


