Question: How do you know this is a steady state? D1 It is desired to enrich the partial pressure of hydrogen in a hydrogen-nitrogen gas mixture
How do you know this is a steady state?
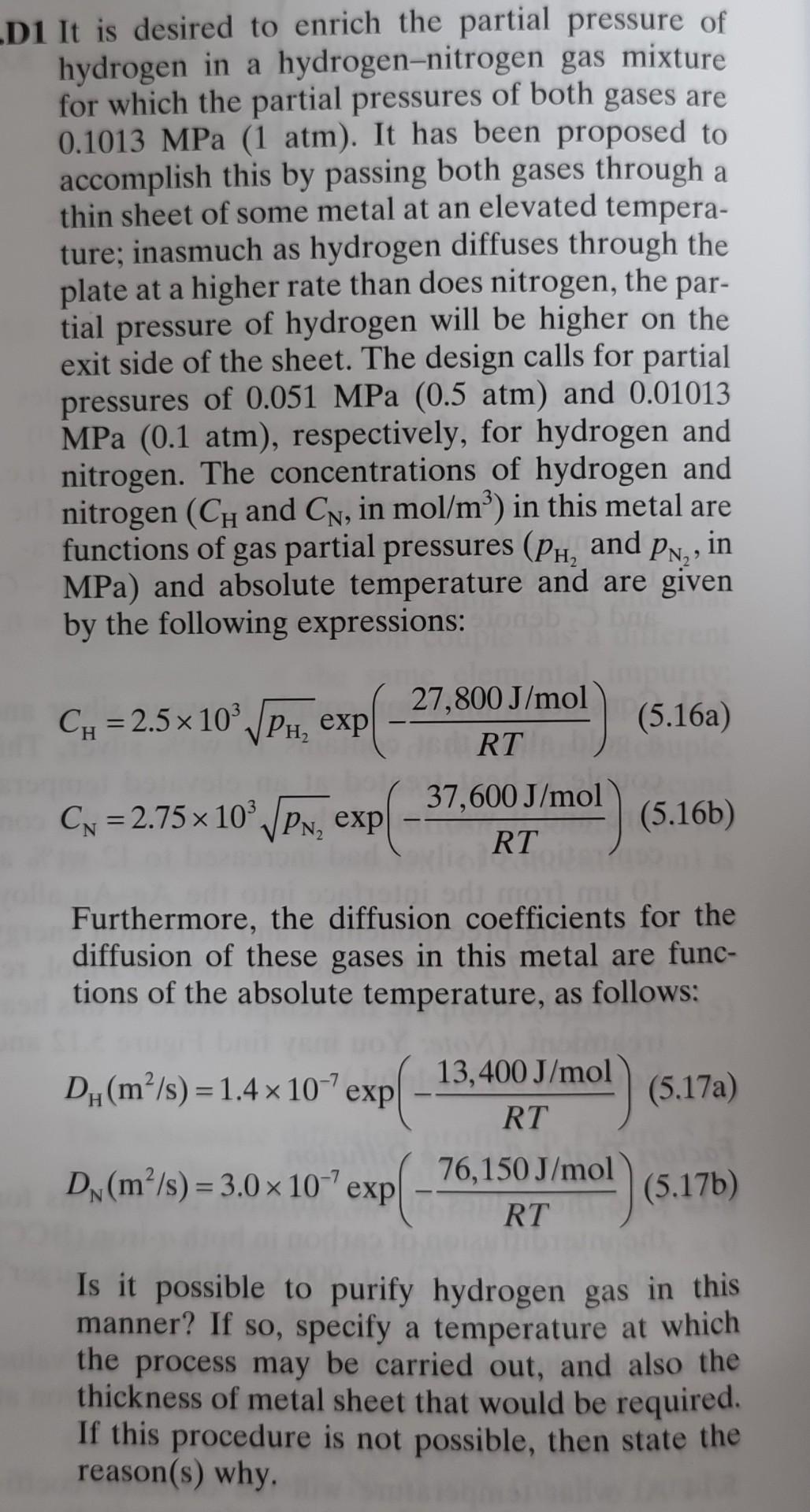
D1 It is desired to enrich the partial pressure of hydrogen in a hydrogen-nitrogen gas mixture for which the partial pressures of both gases are 0.1013MPa(1atm). It has been proposed to accomplish this by passing both gases through a thin sheet of some metal at an elevated temperature; inasmuch as hydrogen diffuses through the plate at a higher rate than does nitrogen, the partial pressure of hydrogen will be higher on the exit side of the sheet. The design calls for partial pressures of 0.051MPa(0.5atm) and 0.01013 MPa ( 0.1atm), respectively, for hydrogen and nitrogen. The concentrations of hydrogen and nitrogen (CH and CN, in mol/m3) in this metal are functions of gas partial pressures (pH2 and pN2, in MPa ) and absolute temperature and are given by the following expressions: CH=2.5103pH2exp(RT27,800J/mol)CN=2.75103pN2exp(RT37,600J/mol) Furthermore, the diffusion coefficients for the diffusion of these gases in this metal are functions of the absolute temperature, as follows: DH(m2/s)=1.4107exp(RT13,400J/mol)DN(m2/s)=3.0107exp(RT76,150J/mol) Is it possible to purify hydrogen gas in this manner? If so, specify a temperature at which the process may be carried out, and also the thickness of metal sheet that would be required. If this procedure is not possible, then state the reason(s) why
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


