Question: How is Table 1 filled with the given data tables above? Fffect Temnerature Tem R ate f Reartion Calculate the molarity of Iin the 250.0mL
How is Table 1 filled with the given data tables above?
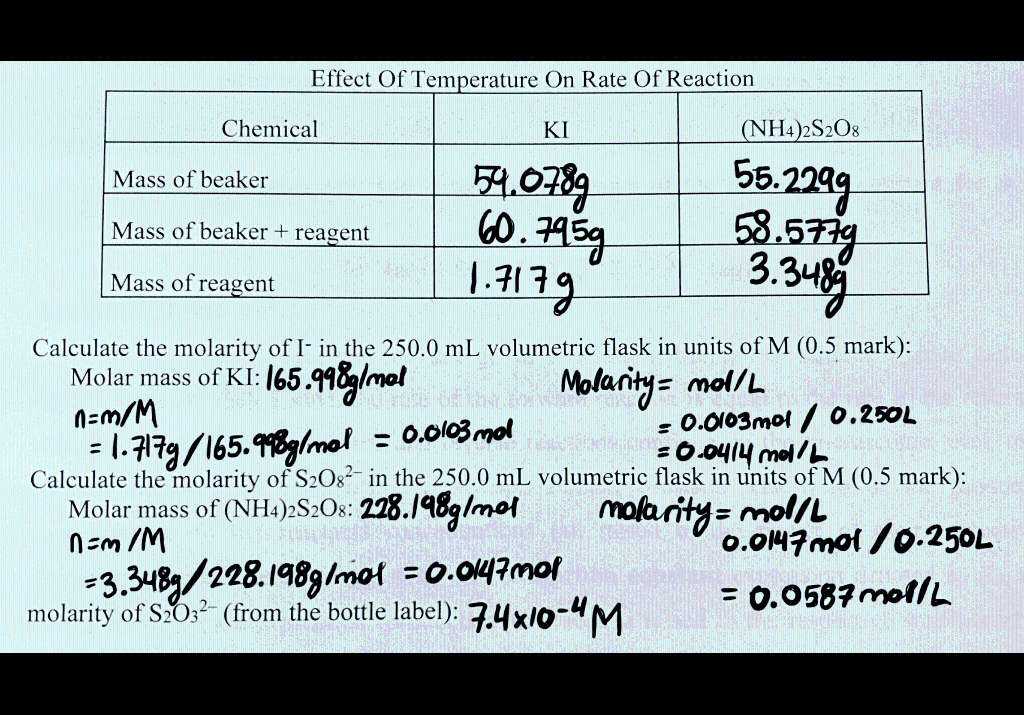
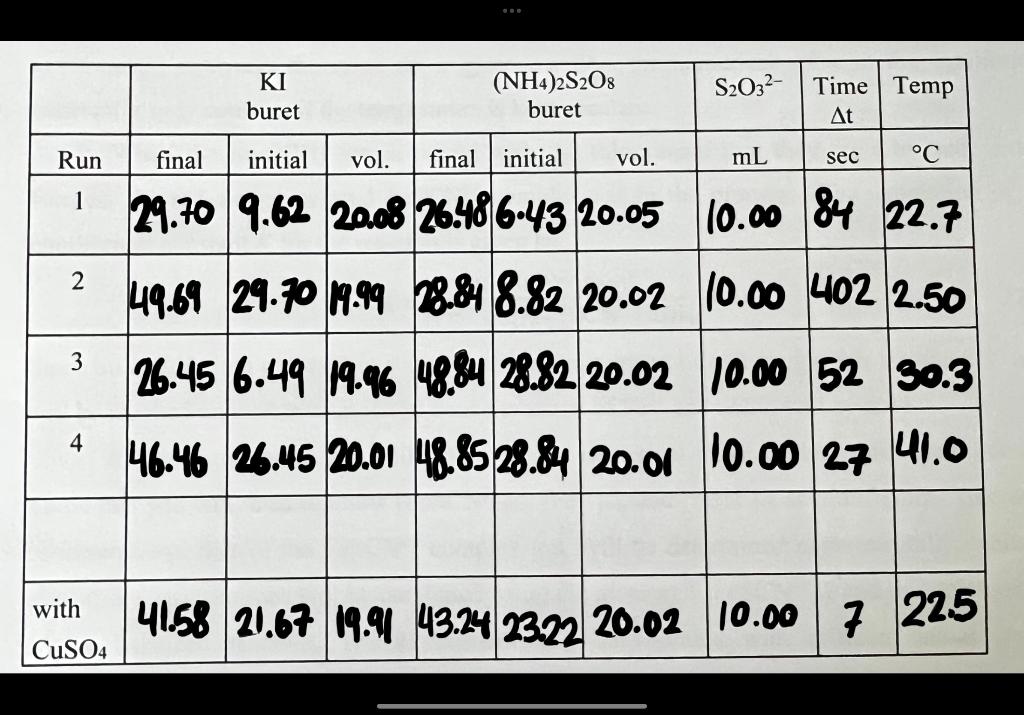
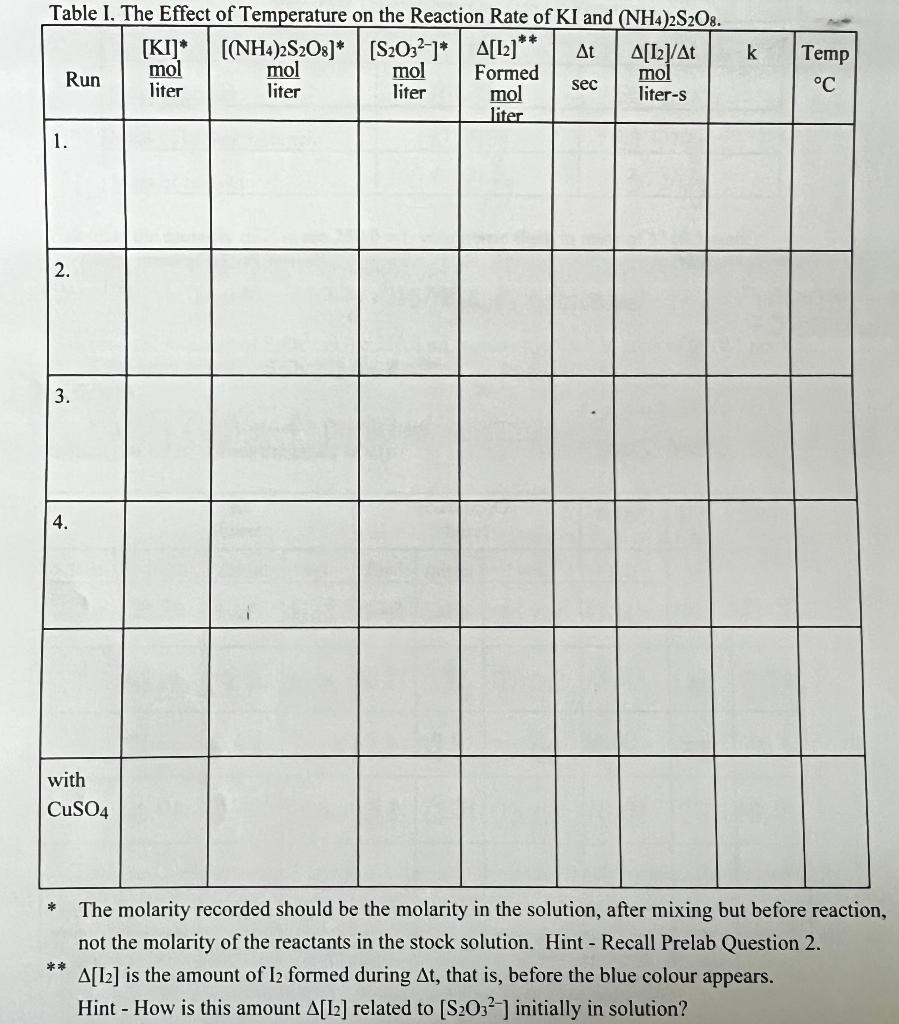
Fffect Temnerature Tem R ate f Reartion Calculate the molarity of Iin the 250.0mL volumetric flask in units of M(0.5 mark): nMolarmassofKI:165.998g/mal=m/M=1.717g/165.96g/mol=0.0103molMolarity=mol/L=0.0103mol/O.250L=0.0414mol/L Calculate the molarity of S2O82 in the 250.0mL volumetric flask in units of M(0.5 mark): not the molarity of the reactants in the stock solution. Hint - Recall Prelab Question 2. [I2] is the amount of I2 formed during t, that is, before the blue colour appears. Hint - How is this amount [L2] related to [S2O32] initially in solution
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


