Question: How long does it take for an N-acetylvaline ethyl ester concentration of 4.8103M to reach a concentration of 7.7104M ? Express your answer in seconds

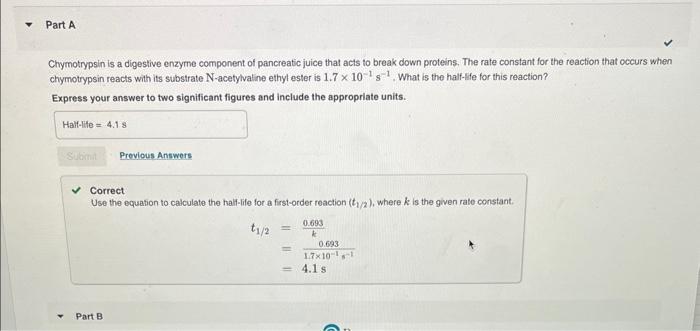

How long does it take for an N-acetylvaline ethyl ester concentration of 4.8103M to reach a concentration of 7.7104M ? Express your answer in seconds to two significant figures. Chymotrypsin is a digestive enzyme component of pancreatic juice that acts to break down proteins. The rate constant for the reaction that occurs when chymotrypsin reacts with its substrate N-acetylvaline ethyl ester is 1.7101s1. What is the half-lfe for this reaction? Express your answer to two significant figures and include the approprlate units. Correct Use the equation to calculate the half-life for a first-order reaction (t1/2), where k is the given rate constant. t1/2=k0.693=1.7101s10.693=4.1s Use the equation to calculate the half-life for a first-order reaction (t1/2), where k is the given rate constant. t1/2=k0.693=1.7101s10.693=4.1s Part B How long does it take for an N-acetylvaline ethyl ester concentration of 4.8103M to reach a concentration of 7.7104M ? Express your answer in seconds to two significant figures
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


