Question: How much does the total angular momentum quantum number J changes in the transition as Cr (3d^6) atom as it ionizes to cr^2t (3d^4)?
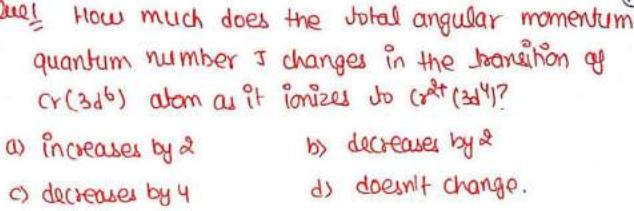
How much does the total angular momentum quantum number J changes in the transition as Cr (3d^6) atom as it ionizes to cr^2t (3d^4)? a) increases by 2 c) decreases by 4 b) decreases by 2 d) doesn't change.
Step by Step Solution
There are 3 Steps involved in it
The detailed ... View full answer

Get step-by-step solutions from verified subject matter experts


