Question: How much (in M) carbon dioxide (CO2) will dissolve in water from air at 10C and 1.8 atm pressure and containing 2% (volume) CO2 ?
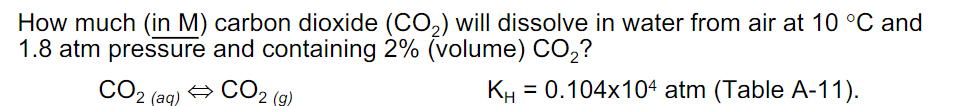
How much (in M) carbon dioxide (CO2) will dissolve in water from air at 10C and 1.8 atm pressure and containing 2% (volume) CO2 ? CO2(aq)CO2(g) KH=0.104104atm( Table A-11)
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


