Question: How much solution could be heated to boiling by the heat evolved by the dissolution of 23.0g of NaOH ? (For the solution, assume a
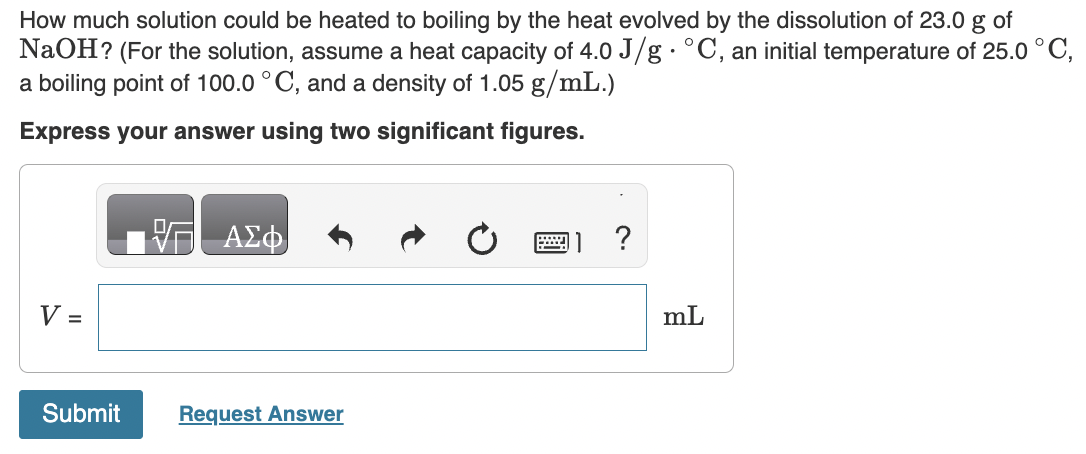
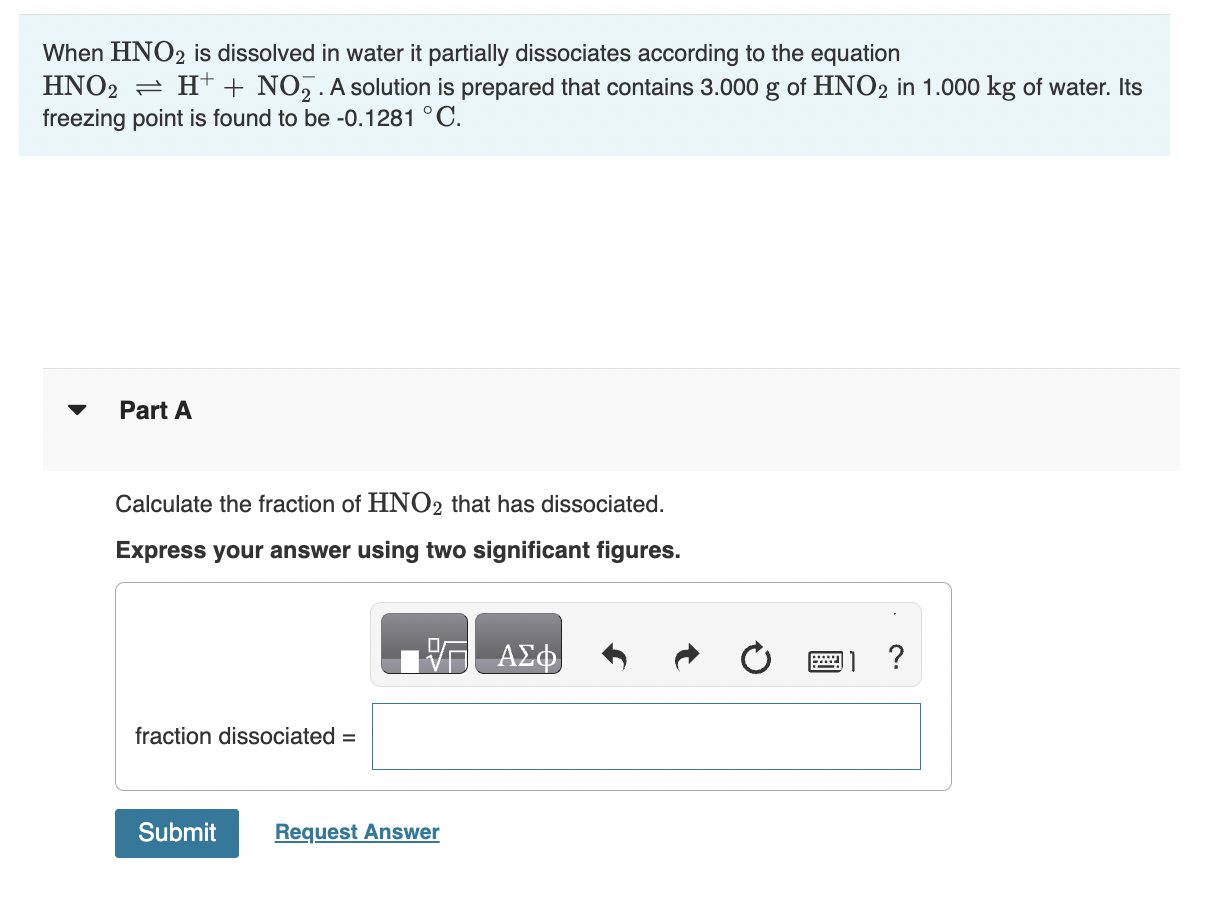
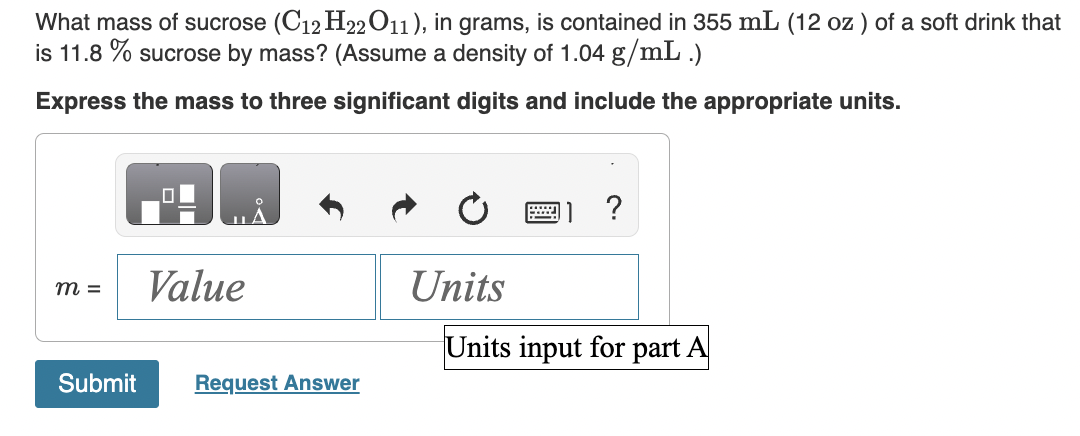
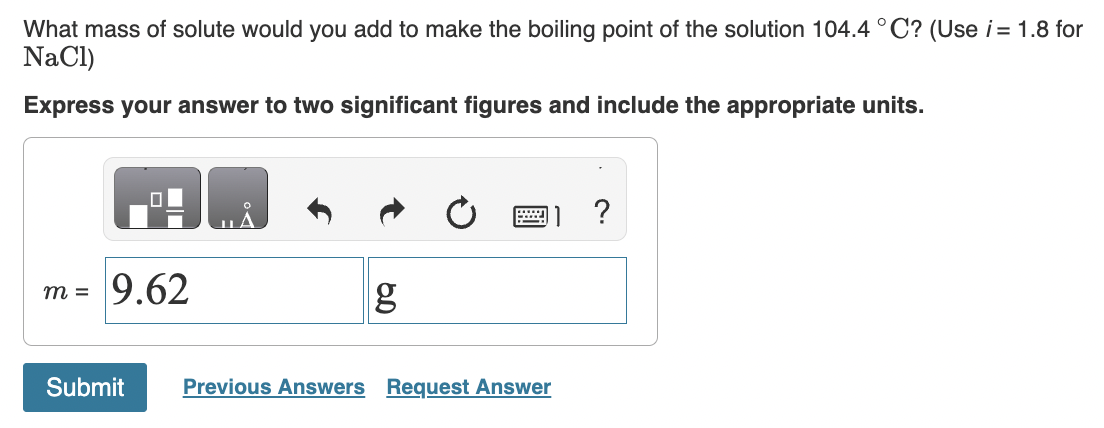
How much solution could be heated to boiling by the heat evolved by the dissolution of 23.0g of NaOH ? (For the solution, assume a heat capacity of 4.0J/gC, an initial temperature of 25.0C, a boiling point of 100.0C, and a density of 1.05g/mL.) Express your answer using two significant figures. When HNO2 is dissolved in water it partially dissociates according to the equation HNO2H++NO2. A solution is prepared that contains 3.000g of HNO2 in 1.000kg of water. Its freezing point is found to be 0.1281C. Part A Calculate the fraction of HNO2 that has dissociated. Express your answer using two significant figures. What mass of sucrose (C12H22O11), in grams, is contained in 355mL(12oz) of a soft drink that is 11.8% sucrose by mass? (Assume a density of 1.04g/mL.) Express the mass to three significant digits and include the appropriate units. What mass of solute would you add to make the boiling point of the solution 104.4C ? (Use i=1.8 for NaCl) Express your answer to two significant figures and include the appropriate units
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


